
Clinical Trial Supply And Logistics Market
Clinical Trial Supply & Logistics Market Forecasts to 2030 - Global Analysis By Phase (Phase I, Phase II, Phase III and Phase IV), By Service Type (Logistics & Distribution, Storage & Retention, Packaging, Labeling, and Blinding and Other Service Types), By Therapeutic Area, Application, End User and By Geography

|
Years Covered |
2021-2030 |
|
Estimated Year Value (2023) |
US $3.9 BN |
|
Projected Year Value (2030) |
US $7.3 BN |
|
CAGR (2023 - 2030) |
9.5% |
|
Regions Covered |
North America, Europe, Asia Pacific, South America, and Middle East & Africa |
|
Countries Covered |
US, Canada, Mexico, Germany, UK, Italy, France, Spain, Japan, China, India, Australia, New Zealand, South Korea, Rest of Asia Pacific, South America, Argentina, Brazil, Chile, Middle East & Africa, Saudi Arabia, UAE, Qatar, and South Africa |
|
Largest Market |
North America |
|
Highest Growing Market |
Asia Pacific |
According to Stratistics MRC, the Global Clinical Trial Supply & Logistics Market is accounted for $3.9 billion in 2023 and is expected to reach $7.3 billion by 2030 growing at a CAGR of 9.5% during the forecast period. Clinical Trial Supply & Logistics (CTSL) is a specialized field ensuring the efficient and secure movement of medication, equipment, and other vital materials needed for clinical research. It encompasses activities like inventory management, packaging, transportation, customs clearance, and temperature control, ensuring timely delivery to clinical trial sites across the globe. CTSL ensures clinical trials run smoothly, contributing to the development of new drugs and therapies.
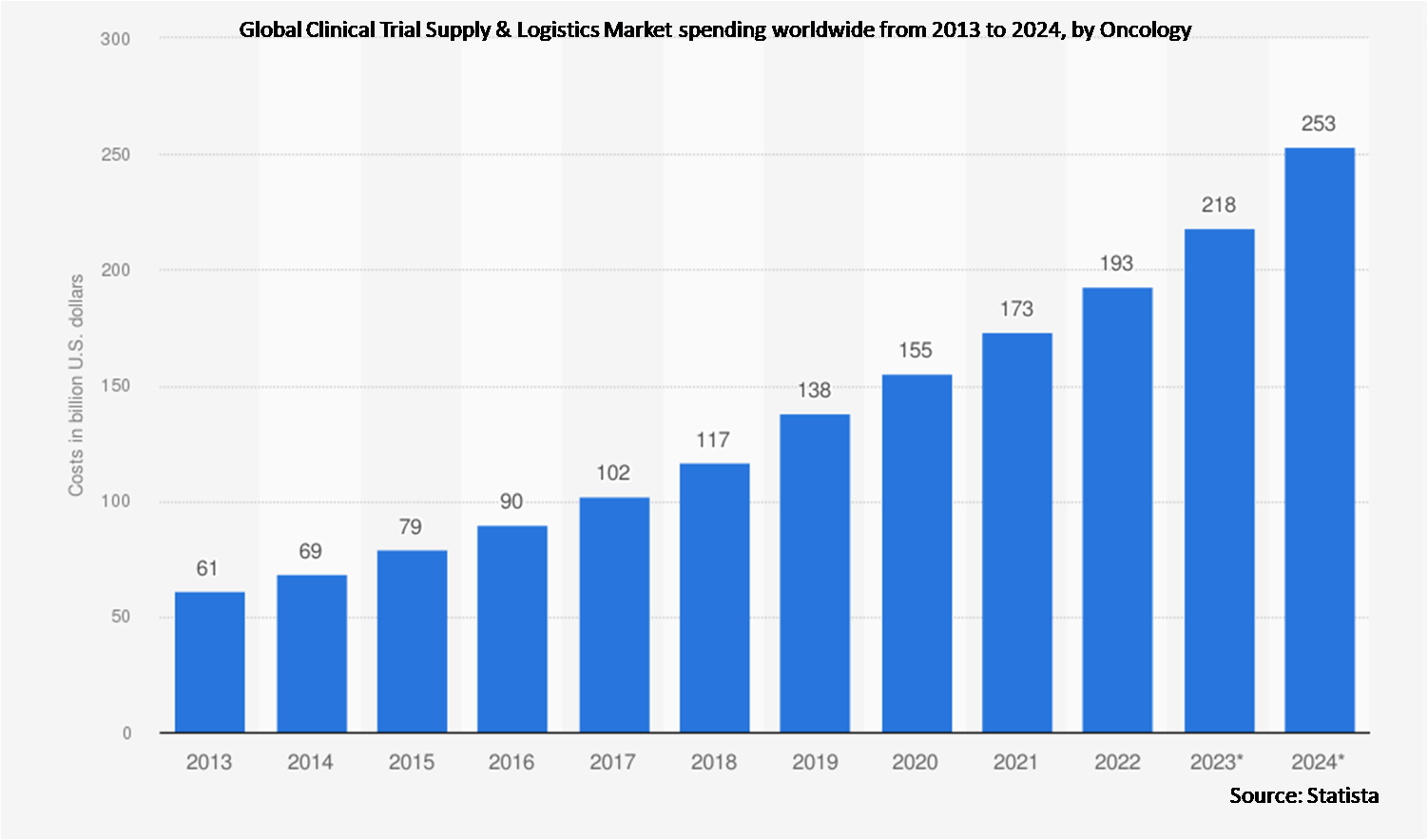
Market Dynamics:
Driver:
Increased demand for clinical trials
The increased demand translates to a booming Clinical Trial Supply & Logistics (CTSL) market. As more trials are conducted, the need for secure transport, precise inventory management, and specialized handling of materials like temperature-sensitive drugs becomes crucial. CTSL providers are stepping up to meet this demand by investing in technology, expanding global reach, and offering customized solutions for personalized medicine and patient engagement. Thus, the booming clinical trial landscape directly fuels the growth and innovation within the CTSL market.
Restraint:
Temperature sensitive products
Clinical trials often involve the transportation and storage of temperature-sensitive drugs, vaccines, or biological samples that require specific and controlled conditions to maintain their efficacy and integrity. The challenge lies in ensuring that these products are consistently maintained within the recommended temperature ranges throughout the supply chain, from manufacturing to final delivery. Any deviation from the specified temperature conditions can compromise the quality and reliability of the clinical trial results, leading to potential safety issues and data inaccuracies.
Opportunity:
The rise of personalized medicine
As treatments increasingly target individual patient profiles, the demand for specialized and customized pharmaceuticals escalates. This paradigm shift necessitates a flexible and efficient supply chain to accommodate diverse trial requirements, ranging from unique drug formulations to specific patient populations. Companies that adeptly adapt their supply and logistics strategies to the intricacies of personalized medicine stand to capitalize on this evolving landscape, driving innovation and enhancing clinical trial success.
Threat:
Public scrutiny and concerns
Any negative perception or controversy surrounding these trials can undermine public trust. Issues such as ethical concerns, patient safety, or perceived corporate interests may trigger intense scrutiny from the media, regulatory bodies, and the general public. Heightened public awareness, fueled by social media and advocacy groups, can amplify the impact of any perceived shortcomings in the supply and logistics processes. Negative perceptions can result in increased regulatory scrutiny, delays in trial timelines, and reputational damage for pharmaceutical companies and research organizations, thereby jeopardizing the overall success of clinical trials and hindering advancements in medical research.
Covid-19 Impact:
The Covid-19 pandemic has significantly impacted the market, introducing challenges such as disrupted supply chains, delays in patient enrollment, and increased demand for certain therapeutic areas. Lockdowns, travel restrictions, and resource reallocation have affected the timely distribution of investigational products, leading to logistical complexities. Pharmaceutical companies and contract research organizations have had to adapt rapidly, emphasizing the importance of resilient supply chain strategies and innovative logistics solutions to ensure the continuity and efficiency of clinical trials during these unprecedented times.
The Phase III segment is expected to be the largest during the forecast period
The Phase III segment is estimated to hold the largest share. The Phase 3 segment of the clinical trials market represents a crucial stage in the development and evaluation of medical interventions, such as drugs, treatments, or therapies. Phase III clinical trials are considered complex clinical trials that require both robust technologies and reliable clinical resources to recruit patients efficiently, initiate sites quickly, and provide cost-effective study management. Furthermore, during Phase 3 trials, researchers carefully gather data to confirm and extend the findings from earlier phases. They aim to demonstrate statistically significant results and compare the intervention against standard treatments, placebos, or other relevant comparators.
The pharmaceuticals segment is expected to have the highest CAGR during the forecast period
During the anticipated period, the pharmaceuticals segment is expected to increase at the most effective rate. This specialized sector focuses on ensuring the timely and secure delivery of investigational drugs to various clinical trial sites. Efficient supply chain logistics, temperature-controlled storage, and regulatory compliance are critical aspects. Companies operating in this market provide comprehensive solutions to streamline the complex process of supplying pharmaceuticals for clinical trials, supporting the development of new drugs and therapies.
Region with largest share:
North America commanded the largest market share during the extrapolated period. The market is expanding in North America due to a number of factors, including the rising incidence of infectious and chronic diseases, the demand for personalised treatment, and the existence of a thriving pharmaceutical sector. The regulatory climate in the area is also supportive, with the FDA playing a key role in the approval and supervision of clinical trials. With an increase in vaccine and therapy studies, the COVID-19 pandemic has also fuelled growth in the North American clinical trials market.
Region with highest CAGR:
Asia-Pacific region is expected to witness profitable growth over the projection period, due to the growing patient base and affordable treatments. The clinical trials market has excellent development potential in Asia and the Pacific. This can be attributed to the region's affordable clinical trials, expanding manufacturing facilities, enacting supportive government regulations, and expanding pharmaceutical business. Cost savings, a large treatment population, participant retention in clinical trials, and ongoing regulatory procedural improvements are mostly responsible for the rise of clinical trial activities in the area.
Key players in the market
Some of the key players in Clinical Trial Supply & Logistics market include Parexel, Thermo Fisher Scientific (Patheon), Catalent, Inc., Packaging Coordinators Inc., Almac Group, Piramal Pharma Solutions, UDG Healthcare, DHL, FedEx and Movianto.
Key Developments:
In October 2023, Piramal Pharma Solutions partnered with an AI-powered platform provider to streamline clinical trial supply chain management. This partnership leverages AI and machine learning to optimize inventory management, route planning, and real-time visibility into the location and status of clinical trial supplies.
In September 2023, Thermo Fisher Scientific (Patheon) acquired a leading European clinical trial logistics company, significantly increasing presence in Europe and expertise in complex trial logistics.
In February 2023, Parexel launched a new expert series, New Medicines, Novel Insights. The series features fresh insights from the company’s global, cross-functional experts analyzing drug development trends and offering evidence-based guidance to the biopharmaceutical industry.
Phases Covered:
• Phase I
• Phase II
• Phase III
• Phase IV
Service Types Covered:
• Logistics & Distribution
• Storage & Retention
• Packaging, Labeling, and Blinding
• Comparator Sourcing
• Other Service Types
Therapeutic Areas Covered:
• Oncology
• Neurology
• Immunology
• Genetic Diseases
• Infectious Diseases
• Metabolic Disorders
• Cardiology
• Other Therapeutic Areas
Applications Covered:
• Small Molecules
• Vaccine
• Monoclonal Antibodies
• Cell & Gene Therapy
• Other Applications
End User Covered:
• Pharmaceuticals
• Biologicals
• Medical Device
• Other End Users
Regions Covered:
• North America
o US
o Canada
o Mexico
• Europe
o Germany
o UK
o Italy
o France
o Spain
o Rest of Europe
• Asia Pacific
o Japan
o China
o India
o Australia
o New Zealand
o South Korea
o Rest of Asia Pacific
• South America
o Argentina
o Brazil
o Chile
o Rest of South America
• Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Rest of Middle East & Africa
What our report offers:
- Market share assessments for the regional and country-level segments
- Strategic recommendations for the new entrants
- Covers Market data for the years 2021, 2022, 2023, 2026, and 2030
- Market Trends (Drivers, Constraints, Opportunities, Threats, Challenges, Investment Opportunities, and recommendations)
- Strategic recommendations in key business segments based on the market estimations
- Competitive landscaping mapping the key common trends
- Company profiling with detailed strategies, financials, and recent developments
- Supply chain trends mapping the latest technological advancements
Free Customization Offerings:
All the customers of this report will be entitled to receive one of the following free customization options:
• Company Profiling
o Comprehensive profiling of additional market players (up to 3)
o SWOT Analysis of key players (up to 3)
• Regional Segmentation
o Market estimations, Forecasts and CAGR of any prominent country as per the client's interest (Note: Depends on feasibility check)
• Competitive Benchmarking
Benchmarking of key players based on product portfolio, geographical presence, and strategic alliances
Table of Contents
1 Executive Summary
2 Preface
2.1 Abstract
2.2 Stake Holders
2.3 Research Scope
2.4 Research Methodology
2.4.1 Data Mining
2.4.2 Data Analysis
2.4.3 Data Validation
2.4.4 Research Approach
2.5 Research Sources
2.5.1 Primary Research Sources
2.5.2 Secondary Research Sources
2.5.3 Assumptions
3 Market Trend Analysis
3.1 Introduction
3.2 Drivers
3.3 Restraints
3.4 Opportunities
3.5 Threats
3.6 Application Analysis
3.7 End User Analysis
3.8 Emerging Markets
3.9 Impact of Covid-19
4 Porters Five Force Analysis
4.1 Bargaining power of suppliers
4.2 Bargaining power of buyers
4.3 Threat of substitutes
4.4 Threat of new entrants
4.5 Competitive rivalry
5 Global Clinical Trial Supply & Logistics Market, By Phase
5.1 Introduction
5.2 Phase I
5.3 Phase II
5.4 Phase III
5.5 Phase IV
6 Global Clinical Trial Supply & Logistics Market, By Service Type
6.1 Introduction
6.2 Logistics & Distribution
6.3 Storage & Retention
6.4 Packaging, Labeling, and Blinding
6.5 Comparator Sourcing
6.6 Other Service Types
7 Global Clinical Trial Supply & Logistics Market, By Therapeutic Area
7.1 Introduction
7.2 Oncology
7.3 Neurology
7.4 Immunology
7.5 Genetic Diseases
7.6 Infectious Diseases
7.7 Metabolic Disorders
7.8 Cardiology
7.9 Other Therapeutic Areas
8 Global Clinical Trial Supply & Logistics Market, By Application
8.1 Introduction
8.2 Small Molecules
8.3 Vaccine
8.4 Monoclonal Antibodies
8.5 Cell & Gene Therapy
8.6 Other Applications
9 Global Clinical Trial Supply & Logistics Market, By End User
9.1 Introduction
9.2 Pharmaceuticals
9.3 Biologicals
9.4 Medical Device
9.5 Other End Users
10 Global Clinical Trial Supply & Logistics Market, By Geography
10.1 Introduction
10.2 North America
10.2.1 US
10.2.2 Canada
10.2.3 Mexico
10.3 Europe
10.3.1 Germany
10.3.2 UK
10.3.3 Italy
10.3.4 France
10.3.5 Spain
10.3.6 Rest of Europe
10.4 Asia Pacific
10.4.1 Japan
10.4.2 China
10.4.3 India
10.4.4 Australia
10.4.5 New Zealand
10.4.6 South Korea
10.4.7 Rest of Asia Pacific
10.5 South America
10.5.1 Argentina
10.5.2 Brazil
10.5.3 Chile
10.5.4 Rest of South America
10.6 Middle East & Africa
10.6.1 Saudi Arabia
10.6.2 UAE
10.6.3 Qatar
10.6.4 South Africa
10.6.5 Rest of Middle East & Africa
11 Key Developments
11.1 Agreements, Partnerships, Collaborations and Joint Ventures
11.2 Acquisitions & Mergers
11.3 New Product Launch
11.4 Expansions
11.5 Other Key Strategies
12 Company Profiling
12.1 Parexel
12.2 Thermo Fisher Scientific (Patheon)
12.3 Catalent, Inc.
12.4 Packaging Coordinators Inc.
12.5 Almac Group
12.6 Piramal Pharma Solutions
12.7 UDG Healthcare
12.8 DHL
12.9 FedEx
12.10 Movianto
List of Tables
1 Global Clinical Trial Supply & Logistics Market Outlook, By Region (2021-2030) ($MN)
2 Global Clinical Trial Supply & Logistics Market Outlook, By Phase (2021-2030) ($MN)
3 Global Clinical Trial Supply & Logistics Market Outlook, By Phase I (2021-2030) ($MN)
4 Global Clinical Trial Supply & Logistics Market Outlook, By Phase II (2021-2030) ($MN)
5 Global Clinical Trial Supply & Logistics Market Outlook, By Phase III (2021-2030) ($MN)
6 Global Clinical Trial Supply & Logistics Market Outlook, By Phase IV (2021-2030) ($MN)
7 Global Clinical Trial Supply & Logistics Market Outlook, By Service Type (2021-2030) ($MN)
8 Global Clinical Trial Supply & Logistics Market Outlook, By Logistics & Distribution (2021-2030) ($MN)
9 Global Clinical Trial Supply & Logistics Market Outlook, By Storage & Retention (2021-2030) ($MN)
10 Global Clinical Trial Supply & Logistics Market Outlook, By Packaging, Labeling, and Blinding (2021-2030) ($MN)
11 Global Clinical Trial Supply & Logistics Market Outlook, By Comparator Sourcing (2021-2030) ($MN)
12 Global Clinical Trial Supply & Logistics Market Outlook, By Other Service Types (2021-2030) ($MN)
13 Global Clinical Trial Supply & Logistics Market Outlook, By Therapeutic Area (2021-2030) ($MN)
14 Global Clinical Trial Supply & Logistics Market Outlook, By Oncology (2021-2030) ($MN)
15 Global Clinical Trial Supply & Logistics Market Outlook, By Neurology (2021-2030) ($MN)
16 Global Clinical Trial Supply & Logistics Market Outlook, By Immunology (2021-2030) ($MN)
17 Global Clinical Trial Supply & Logistics Market Outlook, By Genetic Diseases (2021-2030) ($MN)
18 Global Clinical Trial Supply & Logistics Market Outlook, By Infectious Diseases (2021-2030) ($MN)
19 Global Clinical Trial Supply & Logistics Market Outlook, By Metabolic Disorders (2021-2030) ($MN)
20 Global Clinical Trial Supply & Logistics Market Outlook, By Cardiology (2021-2030) ($MN)
21 Global Clinical Trial Supply & Logistics Market Outlook, By Other Therapeutic Areas (2021-2030) ($MN)
22 Global Clinical Trial Supply & Logistics Market Outlook, By Application (2021-2030) ($MN)
23 Global Clinical Trial Supply & Logistics Market Outlook, By Small Molecules (2021-2030) ($MN)
24 Global Clinical Trial Supply & Logistics Market Outlook, By Vaccine (2021-2030) ($MN)
25 Global Clinical Trial Supply & Logistics Market Outlook, By Monoclonal Antibodies (2021-2030) ($MN)
26 Global Clinical Trial Supply & Logistics Market Outlook, By Cell & Gene Therapy (2021-2030) ($MN)
27 Global Clinical Trial Supply & Logistics Market Outlook, By Other Applications (2021-2030) ($MN)
28 Global Clinical Trial Supply & Logistics Market Outlook, By End User (2021-2030) ($MN)
29 Global Clinical Trial Supply & Logistics Market Outlook, By Pharmaceuticals (2021-2030) ($MN)
30 Global Clinical Trial Supply & Logistics Market Outlook, By Biologicals (2021-2030) ($MN)
31 Global Clinical Trial Supply & Logistics Market Outlook, By Medical Device (2021-2030) ($MN)
32 Global Clinical Trial Supply & Logistics Market Outlook, By Other End Users (2021-2030) ($MN)
Note: Tables for North America, Europe, APAC, South America, and Middle East & Africa Regions are also represented in the same manner as above.
List of Figures
RESEARCH METHODOLOGY
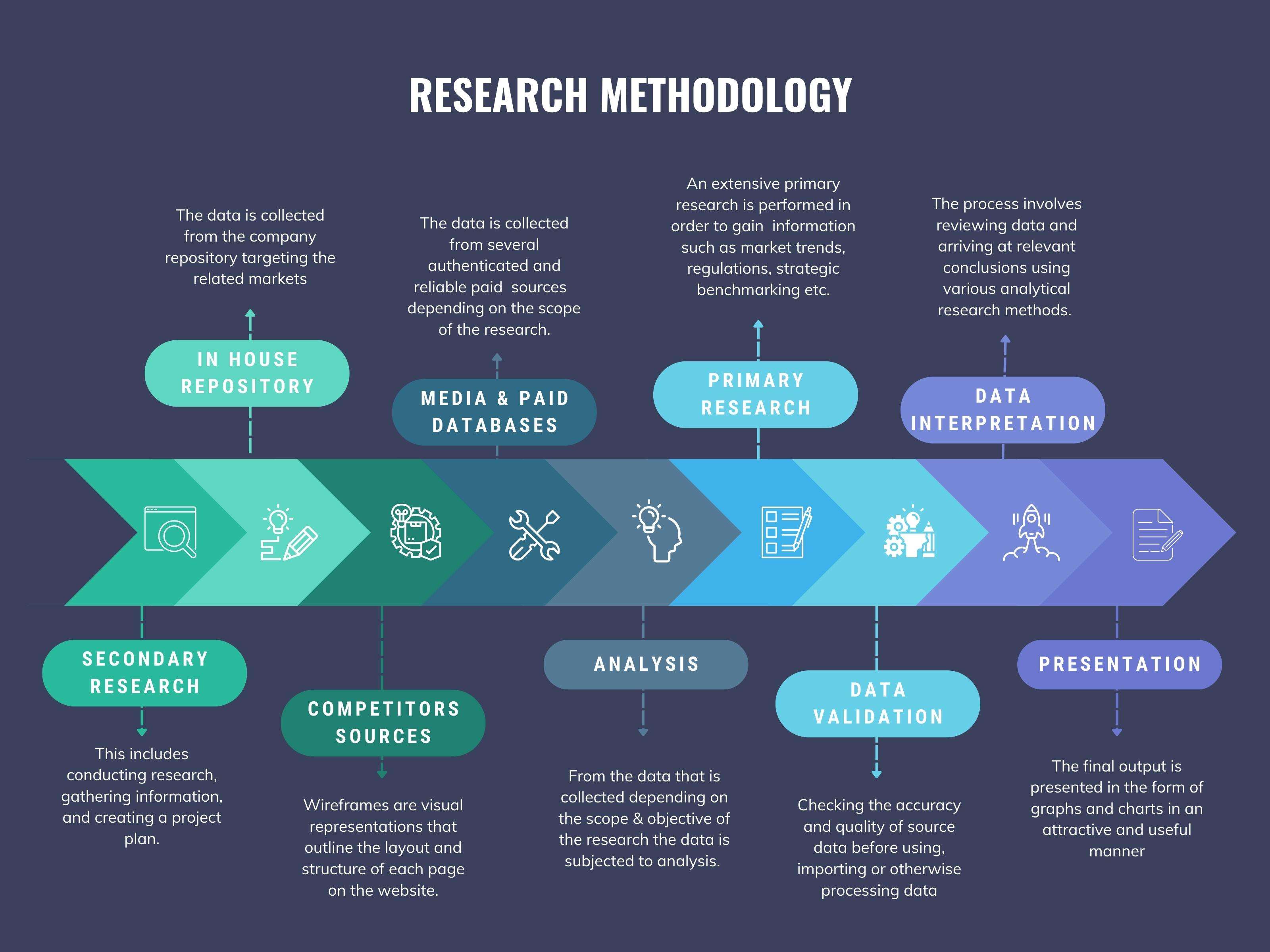
We at ‘Stratistics’ opt for an extensive research approach which involves data mining, data validation, and data analysis. The various research sources include in-house repository, secondary research, competitor’s sources, social media research, client internal data, and primary research.
Our team of analysts prefers the most reliable and authenticated data sources in order to perform the comprehensive literature search. With access to most of the authenticated data bases our team highly considers the best mix of information through various sources to obtain extensive and accurate analysis.
Each report takes an average time of a month and a team of 4 industry analysts. The time may vary depending on the scope and data availability of the desired market report. The various parameters used in the market assessment are standardized in order to enhance the data accuracy.
Data Mining
The data is collected from several authenticated, reliable, paid and unpaid sources and is filtered depending on the scope & objective of the research. Our reports repository acts as an added advantage in this procedure. Data gathering from the raw material suppliers, distributors and the manufacturers is performed on a regular basis, this helps in the comprehensive understanding of the products value chain. Apart from the above mentioned sources the data is also collected from the industry consultants to ensure the objective of the study is in the right direction.
Market trends such as technological advancements, regulatory affairs, market dynamics (Drivers, Restraints, Opportunities and Challenges) are obtained from scientific journals, market related national & international associations and organizations.
Data Analysis
From the data that is collected depending on the scope & objective of the research the data is subjected for the analysis. The critical steps that we follow for the data analysis include:
- Product Lifecycle Analysis
- Competitor analysis
- Risk analysis
- Porters Analysis
- PESTEL Analysis
- SWOT Analysis
The data engineering is performed by the core industry experts considering both the Marketing Mix Modeling and the Demand Forecasting. The marketing mix modeling makes use of multiple-regression techniques to predict the optimal mix of marketing variables. Regression factor is based on a number of variables and how they relate to an outcome such as sales or profits.
Data Validation
The data validation is performed by the exhaustive primary research from the expert interviews. This includes telephonic interviews, focus groups, face to face interviews, and questionnaires to validate our research from all aspects. The industry experts we approach come from the leading firms, involved in the supply chain ranging from the suppliers, distributors to the manufacturers and consumers so as to ensure an unbiased analysis.
We are in touch with more than 15,000 industry experts with the right mix of consultants, CEO's, presidents, vice presidents, managers, experts from both supply side and demand side, executives and so on.
The data validation involves the primary research from the industry experts belonging to:
- Leading Companies
- Suppliers & Distributors
- Manufacturers
- Consumers
- Industry/Strategic Consultants
Apart from the data validation the primary research also helps in performing the fill gap research, i.e. providing solutions for the unmet needs of the research which helps in enhancing the reports quality.
For more details about research methodology, kindly write to us at info@strategymrc.com
Frequently Asked Questions
In case of any queries regarding this report, you can contact the customer service by filing the “Inquiry Before Buy” form available on the right hand side. You may also contact us through email: info@strategymrc.com or phone: +1-301-202-5929
Yes, the samples are available for all the published reports. You can request them by filling the “Request Sample” option available in this page.
Yes, you can request a sample with your specific requirements. All the customized samples will be provided as per the requirement with the real data masked.
All our reports are available in Digital PDF format. In case if you require them in any other formats, such as PPT, Excel etc you can submit a request through “Inquiry Before Buy” form available on the right hand side. You may also contact us through email: info@strategymrc.com or phone: +1-301-202-5929
We offer a free 15% customization with every purchase. This requirement can be fulfilled for both pre and post sale. You may send your customization requirements through email at info@strategymrc.com or call us on +1-301-202-5929.
We have 3 different licensing options available in electronic format.
- Single User Licence: Allows one person, typically the buyer, to have access to the ordered product. The ordered product cannot be distributed to anyone else.
- 2-5 User Licence: Allows the ordered product to be shared among a maximum of 5 people within your organisation.
- Corporate License: Allows the product to be shared among all employees of your organisation regardless of their geographical location.
All our reports are typically be emailed to you as an attachment.
To order any available report you need to register on our website. The payment can be made either through CCAvenue or PayPal payments gateways which accept all international cards.
We extend our support to 6 months post sale. A post sale customization is also provided to cover your unmet needs in the report.
Request Customization
We provide a free 15% customization on every purchase. This requirement can be fulfilled for both pre and post sale. You may send your customization requirements through email at info@strategymrc.com or call us on +1-301-202-5929.
Note: This customization is absolutely free until it falls under the 15% bracket. If your requirement exceeds this a feasibility check will be performed. Post that, a quote will be provided along with the timelines.
WHY CHOOSE US ?

Assured Quality
Best in class reports with high standard of research integrity

24X7 Research Support
Continuous support to ensure the best customer experience.

Free Customization
Adding more values to your product of interest.

Safe & Secure Access
Providing a secured environment for all online transactions.

Trusted by 600+ Brands
Serving the most reputed brands across the world.
