
Contract Development And Manufacturing Organization Cdmo Market
Contract Development and Manufacturing Organization (CDMO) Market Forecasts to 2030 - Global Analysis By Contract Manufacturing (Active Pharmaceutical Ingredient (API), Finished Dosage Formulation (FDF), Packaging and Other Contract Manufacturings), Contract Development, End User and By Geography

According to Stratistics MRC, the Global Contract Development and Manufacturing Organization (CDMO) Market is accounted for $259.4 billion in 2023 and is expected to reach $555.8 billion by 2030 growing at a CAGR of 11.5% during the forecast period. An organization known as a Contract Development and Manufacturing Organization (or CDMO) offers specialized services to the biotechnology, pharmaceutical, and medical device sectors. These companies provide contract-based complete services including pharmaceutical product research, manufacture, and occasionally commercialization. They could serve both big and small businesses that need capacity, infrastructure, or knowledge that they might not have on staff.
According to the World Health Organization (WHO), the proportion of the burden of non-communicable diseases (NCDs) is anticipated to reach to 57% in 2020.
Market Dynamics:
Driver:
Increasing need for pharmaceuticals
The increasing global population, increased acceptance of westernized lifestyles, and accelerating economic growth are all contributing to a notable increase in the burden of chronic illnesses. Pharmaceutical businesses are being encouraged to create efficient pharmaceutical goods to speed up the treatment procedure by the rising incidence of chronic illnesses and the aging population. Furthermore, the ability to streamline the pharmaceutical supply chain and the implementation of the one-stop-shop concept to provide efficacious medicinal goods into the market are anticipated to drive the market's expansion.
Restraint:
Stringent regulatory requirements
Comprehensive documentation is required by regulatory organizations at every stage of the product life cycle, from development and manufacture to distribution and production. This results in a significant administrative load and makes it necessary to maintain thorough and accurate records, which can take a lot of time and resources. The key elements that are most likely to impede the market's growth are the existence of stringent government restrictions and a decline in the number of small molecules and biologics that are approved in developed countries.
Opportunity:
Growing use of analytics
CDMOs can employ a wide range of analytical methods and technologies. By employing forecast analytics, charts, percentage change analytics, and numerical analytics to find significant trends in the data, businesses may increase production and efficiency. Because analytics must be used in a dynamic framework and in real-time, it can be a challenging procedure. This solution also aids in the creation and use of techniques that might improve the productivity of a product's development process. Throughout the projected period, the market will expand as a result of the increasing usage of analytics.
Threat:
Cost and time implications
A significant financial commitment as well as extra time are frequently needed for paperwork, quality control methods, employee training, infrastructure upgrades to match changing requirements, and staff training in order to maintain compliance. This may have a substantial effect on the total cost and duration of product development and production. Political actions, changes in the market environment, or advances in science can all lead to frequent changes in regulatory standards. This is the element impeding the market's expansion.
Covid-19 Impact:
The COVID-19 pandemic has had a lasting effect on a number of sectors and has had a major influence on the world economy. Nonetheless, this pandemic had a favorable effect on the healthcare contract development and manufacturing organization sector. The small hospitals, clinics and nursing homes, have been asked and enforced to shut their operations. As a result of social distancing reduction in number of elective surgical procedures during the pandemic, in order to avoid the hospital acquired infections. The number of outsourced manufacturing agreements has grown because to the unexpected spike in demand for vaccines, which is boosting the market's growth.
The packaging segment is expected to be the largest during the forecast period
The packaging segment is expected to be the largest during the forecast period. By including dosage monitoring elements in packaging, the use of patient-centric packaging innovations by pharmaceutical contract manufacturing businesses has emerged as a critical component in enhancing patient adherence. Furthermore, the quick cooperation between different CDMOs to offer creative packaging solutions could increase demand for the packaging segment during the course of the projected period.
The pharmaceutical segment is expected to have the highest CAGR during the forecast period
The pharmaceutical segment is expected to have the highest CAGR during the forecast period. Modern facilities, specialized knowledge, and experience are common assets of CDMOs that are committed to pharmaceutical development. Their concentration on certain facets of medication production, such manufacturing, process optimization, analytical testing, and formulation, enables a breadth of expertise and understanding that may be highly advantageous to pharmaceutical firms.
Region with largest share:
North America is projected to hold the largest market share during the forecast period. It is distinguished by the existence of several well-known biotechnology, pharmaceutical, and medical device businesses. The region's strong demand for effective healthcare is predicted to drive rapid growth in the medical device manufacturing industry, which is anticipated to be one of the key drivers of market expansion.
Region with highest CAGR:
Asia Pacific is projected to hold the highest CAGR over the forecast period due to high healthcare expenditure and rapid introduction of advanced medical devices. The market is anticipated to be driven by elements such an enhanced regulatory environment, significant potential for cost savings, more sophisticated product designs, heightened regulatory emphasis on manufacturing quality control, and a growth in the number of medical device businesses operating in the region.
Key players in the market
Some of the key players in Contract Development and Manufacturing Organization (CDMO) market include Patheon N.V., Cambrex Corporation, Boehringer Ingelheim GmbH, Catalent, Inc., Thermo Fisher Scientific, Inc., Lonza Group Ltd., Evonik Industries AG, Vetter Pharma International GmbH, AbbVie Contract Manufacturing, Fujifilm Holdings Corporation, Samsung Biologics Co., Ltd., Jubilant Life Sciences Limited, Piramal Pharma Solutions, Almac Group Limited, Cytovance Biologics, Inc., Biocon Limited, Grifols SA, Ajinomoto Bio-Pharma Services and Recipharm AB.
Key Developments:
In June 2023, Catalent announced that it has broadened the scope of its One Bio Suite solution, comprising development, manufacturing, and supply for a range of biotechnological modalities including antibody and recombinant proteins, cellular and gene therapy as well as mRNA.
In January 2023, Catalent announced that it signed a development and license agreement with Ethicann Pharmaceuticals Inc., a Canadian/American specialty pharmaceutical company specializing in creating high-value cannabinoid drug therapies, using Zydis orally disintegrating tablet (ODT) technology to advance Ethicann's clinical drug pipeline.
In January 2023, Thermo Fisher Scientific Inc. acquired the global provider of specialty diagnostics, Binding Site Group, for USD 2.8 billion. With the addition of ground-breaking innovation in multiple myeloma diagnosis and monitoring, the Binding Site broadens Thermo Fisher's already expanded specialized diagnostics range.
Contract Manufacturings Covered:
• Active Pharmaceutical Ingredient (API)
• Finished Dosage Formulation (FDF)
• Packaging
• Other Contract Manufacturings
Contract Developments Covered:
• Pre-clinical
• Clinical
• Laboratory Services
• Discovery
• Other Contract Developments
End Users Covered:
• Pharmaceutical
• Biotechnology
• Healthcare
• Personal Care
• Nutraceutical
• Chemical
• Other End Users
Regions Covered:
• North America
o US
o Canada
o Mexico
• Europe
o Germany
o UK
o Italy
o France
o Spain
o Rest of Europe
• Asia Pacific
o Japan
o China
o India
o Australia
o New Zealand
o South Korea
o Rest of Asia Pacific
• South America
o Argentina
o Brazil
o Chile
o Rest of South America
• Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Rest of Middle East & Africa
What our report offers:
- Market share assessments for the regional and country-level segments
- Strategic recommendations for the new entrants
- Covers Market data for the years 2021, 2022, 2023, 2026, and 2030
- Market Trends (Drivers, Constraints, Opportunities, Threats, Challenges, Investment Opportunities, and recommendations)
- Strategic recommendations in key business segments based on the market estimations
- Competitive landscaping mapping the key common trends
- Company profiling with detailed strategies, financials, and recent developments
- Supply chain trends mapping the latest technological advancements
Free Customization Offerings:
All the customers of this report will be entitled to receive one of the following free customization options:
• Company Profiling
o Comprehensive profiling of additional market players (up to 3)
o SWOT Analysis of key players (up to 3)
• Regional Segmentation
o Market estimations, Forecasts and CAGR of any prominent country as per the client's interest (Note: Depends on feasibility check)
• Competitive Benchmarking
o Benchmarking of key players based on product portfolio, geographical presence, and strategic alliances
Table of Contents
1 Executive Summary
2 Preface
2.1 Abstract
2.2 Stake Holders
2.3 Research Scope
2.4 Research Methodology
2.4.1 Data Mining
2.4.2 Data Analysis
2.4.3 Data Validation
2.4.4 Research Approach
2.5 Research Sources
2.5.1 Primary Research Sources
2.5.2 Secondary Research Sources
2.5.3 Assumptions
3 Market Trend Analysis
3.1 Introduction
3.2 Drivers
3.3 Restraints
3.4 Opportunities
3.5 Threats
3.6 End User Analysis
3.7 Emerging Markets
3.8 Impact of Covid-19
4 Porters Five Force Analysis
4.1 Bargaining power of suppliers
4.2 Bargaining power of buyers
4.3 Threat of substitutes
4.4 Threat of new entrants
4.5 Competitive rivalry
5 Global Contract Development and Manufacturing Organization (CDMO) Market, By Contract Manufacturing
5.1 Introduction
5.2 Active Pharmaceutical Ingredient (API)
5.2.1 High Potency
5.2.2 Large Molecule
5.2.3 Small Molecule
5.3 Finished Dosage Formulation (FDF)
5.3.1 Solid Dose Formulation
5.3.2 Liquid Dose Formulation
5.3.3 Injectable Dose Formulation
5.4 Packaging
5.5 Other Contract Manufacturings
6 Global Contract Development and Manufacturing Organization (CDMO) Market, By Contract Development
6.1 Introduction
6.2 Pre-clinical
6.2.1 Toxicology Testing
6.2.2 Bioanalysis
6.3 Clinical
6.3.1 Phase I
6.3.2 Phase II
6.3.3 Phase III
6.3.4 Phase IV
6.4 Laboratory Services
6.4.1 Analytical Services
6.4.2 Bioanalytical Services
6.5 Discovery
6.6 Other Contract Developments
7 Global Contract Development and Manufacturing Organization (CDMO) Market, By End User
7.1 Introduction
7.2 Pharmaceutical
7.3 Biotechnology
7.4 Healthcare
7.5 Personal Care
7.6 Nutraceutical
7.7 Chemical
7.8 Other End Users
8 Global Contract Development and Manufacturing Organization (CDMO) Market, By Geography
8.1 Introduction
8.2 North America
8.2.1 US
8.2.2 Canada
8.2.3 Mexico
8.3 Europe
8.3.1 Germany
8.3.2 UK
8.3.3 Italy
8.3.4 France
8.3.5 Spain
8.3.6 Rest of Europe
8.4 Asia Pacific
8.4.1 Japan
8.4.2 China
8.4.3 India
8.4.4 Australia
8.4.5 New Zealand
8.4.6 South Korea
8.4.7 Rest of Asia Pacific
8.5 South America
8.5.1 Argentina
8.5.2 Brazil
8.5.3 Chile
8.5.4 Rest of South America
8.6 Middle East & Africa
8.6.1 Saudi Arabia
8.6.2 UAE
8.6.3 Qatar
8.6.4 South Africa
8.6.5 Rest of Middle East & Africa
9 Key Developments
9.1 Agreements, Partnerships, Collaborations and Joint Ventures
9.2 Acquisitions & Mergers
9.3 New Product Launch
9.4 Expansions
9.5 Other Key Strategies
10 Company Profiling
10.1 Patheon N.V.
10.2 Cambrex Corporation
10.3 Boehringer Ingelheim GmbH
10.4 Catalent, Inc.
10.5 Thermo Fisher Scientific, Inc.
10.6 Lonza Group Ltd.
10.7 Evonik Industries AG
10.8 Vetter Pharma International GmbH
10.9 AbbVie Contract Manufacturing
10.10 Fujifilm Holdings Corporation
10.11 Samsung Biologics Co., Ltd.
10.12 Jubilant Life Sciences Limited
10.13 Piramal Pharma Solutions
10.14 Almac Group Limited
10.15 Cytovance Biologics, Inc.
10.16 Biocon Limited
10.17 Grifols SA
10.18 Ajinomoto Bio-Pharma Services
10.19 Recipharm AB
List of Tables
1 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Region (2021-2030) ($MN)
2 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Contract Manufacturing (2021-2030) ($MN) 3 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Active Pharmaceutical Ingredient (API) (2021-2030) ($MN)
4 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By High Potency (2021-2030) ($MN)
5 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Large Molecule (2021-2030) ($MN)
6 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Small Molecule (2021-2030) ($MN)
7 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Finished Dosage Formulation (FDF) (2021-2030) ($MN)
8 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Solid Dose Formulation (2021-2030) ($MN)
9 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Liquid Dose Formulation (2021-2030) ($MN)
10 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Injectable Dose Formulation (2021-2030) ($MN)
11 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Packaging (2021-2030) ($MN)
12 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Other Contract Manufacturings (2021-2030) ($MN)
13 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Contract Development (2021-2030) ($MN)
14 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Pre-clinical (2021-2030) ($MN)
15 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Toxicology Testing (2021-2030) ($MN)
16 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Bioanalysis (2021-2030) ($MN)
17 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Clinical (2021-2030) ($MN)
18 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Phase I (2021-2030) ($MN)
19 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Phase II (2021-2030) ($MN)
20 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Phase III (2021-2030) ($MN)
21 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Phase IV (2021-2030) ($MN)
22 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Laboratory Services (2021-2030) ($MN)
23 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Analytical Services (2021-2030) ($MN)
24 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Bioanalytical Services (2021-2030) ($MN)
25 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Discovery (2021-2030) ($MN)
26 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Other Contract Developments (2021-2030) ($MN)
27 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By End User (2021-2030) ($MN)
28 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Pharmaceutical (2021-2030) ($MN)
29 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Biotechnology (2021-2030) ($MN)
30 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Healthcare (2021-2030) ($MN)
31 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Personal Care (2021-2030) ($MN)
32 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Nutraceutical (2021-2030) ($MN)
33 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Chemical (2021-2030) ($MN)
34 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook, By Other End Users (2021-2030) ($MN)
Note: Tables for North America, Europe, APAC, South America, and Middle East & Africa Regions are also represented in the same manner as above.
List of Figures
List of Figures
Figure 1 Global Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 2 North America Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 3 US Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 4 Canada Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 5 Mexico Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 6 Europe Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 7 Germany Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 8 UK Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 9 Italy Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 10 France Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 11 Spain Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 12 Rest of Europe Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 13 Asia Pacific Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 14 Japan Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 15 China Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 16 India Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 17 Australia Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 18 New Zealand Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 19 South Korea Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 20 Rest of Asia Pacific Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 21 South America Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 22 Argentina Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 23 Brazil Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 24 Chile Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 25 Rest of South America Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 26 Middle East & Africa Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 27 Saudi Arabia Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 28 UAE Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 29 Qatar Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 30 South Africa Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
Figure 31 Rest of Middle East & Africa Contract Development and Manufacturing Organization (CDMO) Market Outlook (2021-2030) ($MN)
RESEARCH METHODOLOGY
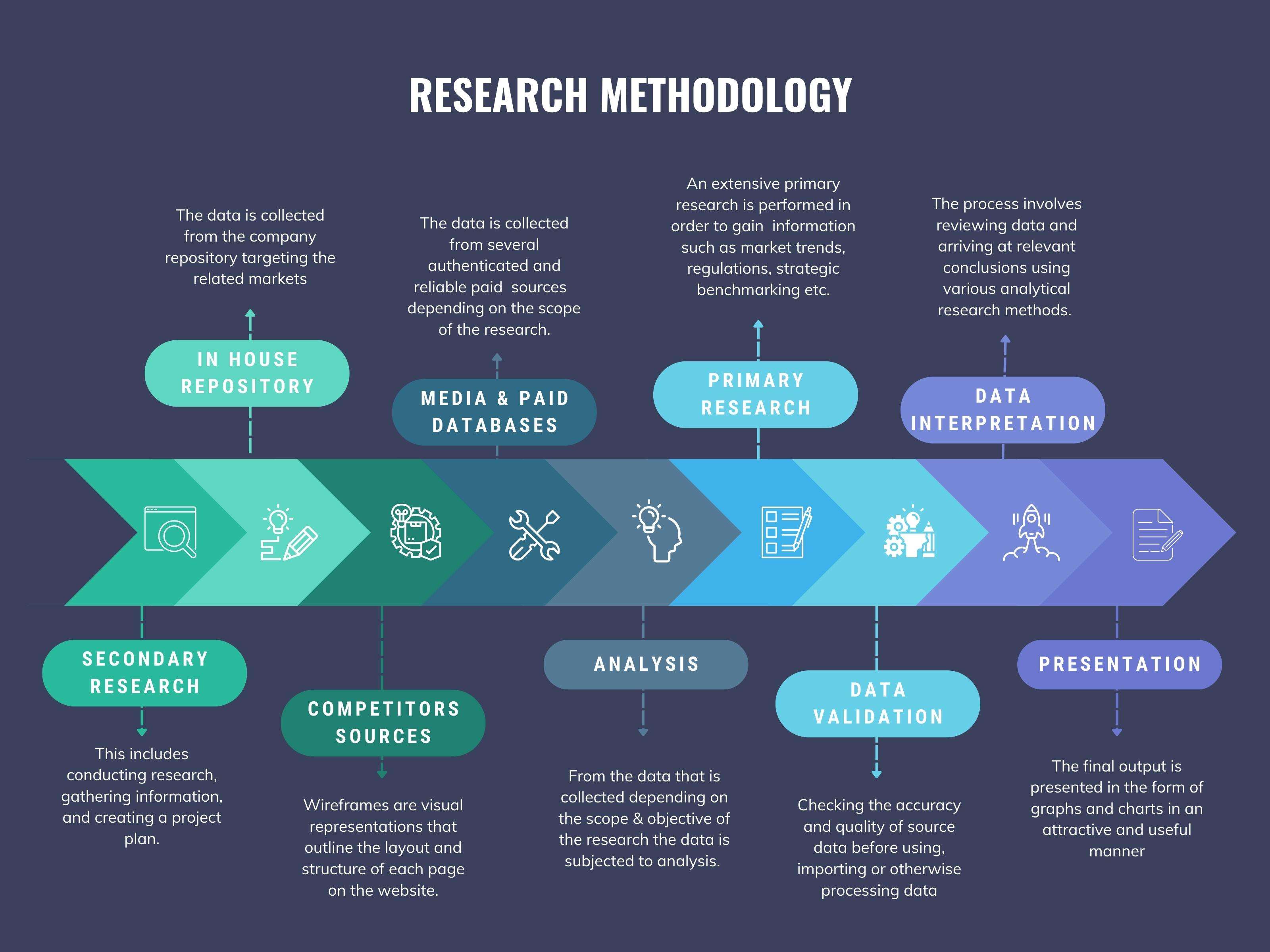
We at ‘Stratistics’ opt for an extensive research approach which involves data mining, data validation, and data analysis. The various research sources include in-house repository, secondary research, competitor’s sources, social media research, client internal data, and primary research.
Our team of analysts prefers the most reliable and authenticated data sources in order to perform the comprehensive literature search. With access to most of the authenticated data bases our team highly considers the best mix of information through various sources to obtain extensive and accurate analysis.
Each report takes an average time of a month and a team of 4 industry analysts. The time may vary depending on the scope and data availability of the desired market report. The various parameters used in the market assessment are standardized in order to enhance the data accuracy.
Data Mining
The data is collected from several authenticated, reliable, paid and unpaid sources and is filtered depending on the scope & objective of the research. Our reports repository acts as an added advantage in this procedure. Data gathering from the raw material suppliers, distributors and the manufacturers is performed on a regular basis, this helps in the comprehensive understanding of the products value chain. Apart from the above mentioned sources the data is also collected from the industry consultants to ensure the objective of the study is in the right direction.
Market trends such as technological advancements, regulatory affairs, market dynamics (Drivers, Restraints, Opportunities and Challenges) are obtained from scientific journals, market related national & international associations and organizations.
Data Analysis
From the data that is collected depending on the scope & objective of the research the data is subjected for the analysis. The critical steps that we follow for the data analysis include:
- Product Lifecycle Analysis
- Competitor analysis
- Risk analysis
- Porters Analysis
- PESTEL Analysis
- SWOT Analysis
The data engineering is performed by the core industry experts considering both the Marketing Mix Modeling and the Demand Forecasting. The marketing mix modeling makes use of multiple-regression techniques to predict the optimal mix of marketing variables. Regression factor is based on a number of variables and how they relate to an outcome such as sales or profits.
Data Validation
The data validation is performed by the exhaustive primary research from the expert interviews. This includes telephonic interviews, focus groups, face to face interviews, and questionnaires to validate our research from all aspects. The industry experts we approach come from the leading firms, involved in the supply chain ranging from the suppliers, distributors to the manufacturers and consumers so as to ensure an unbiased analysis.
We are in touch with more than 15,000 industry experts with the right mix of consultants, CEO's, presidents, vice presidents, managers, experts from both supply side and demand side, executives and so on.
The data validation involves the primary research from the industry experts belonging to:
- Leading Companies
- Suppliers & Distributors
- Manufacturers
- Consumers
- Industry/Strategic Consultants
Apart from the data validation the primary research also helps in performing the fill gap research, i.e. providing solutions for the unmet needs of the research which helps in enhancing the reports quality.
For more details about research methodology, kindly write to us at info@strategymrc.com
Frequently Asked Questions
In case of any queries regarding this report, you can contact the customer service by filing the “Inquiry Before Buy” form available on the right hand side. You may also contact us through email: info@strategymrc.com or phone: +1-301-202-5929
Yes, the samples are available for all the published reports. You can request them by filling the “Request Sample” option available in this page.
Yes, you can request a sample with your specific requirements. All the customized samples will be provided as per the requirement with the real data masked.
All our reports are available in Digital PDF format. In case if you require them in any other formats, such as PPT, Excel etc you can submit a request through “Inquiry Before Buy” form available on the right hand side. You may also contact us through email: info@strategymrc.com or phone: +1-301-202-5929
We offer a free 15% customization with every purchase. This requirement can be fulfilled for both pre and post sale. You may send your customization requirements through email at info@strategymrc.com or call us on +1-301-202-5929.
We have 3 different licensing options available in electronic format.
- Single User Licence: Allows one person, typically the buyer, to have access to the ordered product. The ordered product cannot be distributed to anyone else.
- 2-5 User Licence: Allows the ordered product to be shared among a maximum of 5 people within your organisation.
- Corporate License: Allows the product to be shared among all employees of your organisation regardless of their geographical location.
All our reports are typically be emailed to you as an attachment.
To order any available report you need to register on our website. The payment can be made either through CCAvenue or PayPal payments gateways which accept all international cards.
We extend our support to 6 months post sale. A post sale customization is also provided to cover your unmet needs in the report.
Request Customization
We provide a free 15% customization on every purchase. This requirement can be fulfilled for both pre and post sale. You may send your customization requirements through email at info@strategymrc.com or call us on +1-301-202-5929.
Note: This customization is absolutely free until it falls under the 15% bracket. If your requirement exceeds this a feasibility check will be performed. Post that, a quote will be provided along with the timelines.
WHY CHOOSE US ?

Assured Quality
Best in class reports with high standard of research integrity

24X7 Research Support
Continuous support to ensure the best customer experience.

Free Customization
Adding more values to your product of interest.

Safe & Secure Access
Providing a secured environment for all online transactions.

Trusted by 600+ Brands
Serving the most reputed brands across the world.
