
Influenza Diagnostic Tests Market
Influenza Diagnostic Tests Market Forecasts to 2030 - Global Analysis By Test Type (Molecular Diagnostic Assay, Traditional Diagnostic Test and Other Test Types), End User (Research Laboratories, Hospitals, Diagnostics Centers and Other End Users), and By Geography

|
Years Covered |
2021-2030 |
|
Estimated Year Value (2023) |
US $1.1 BN |
|
Projected Year Value (2030) |
US $1.7 BN |
|
CAGR (2023 - 2030) |
5.6% |
|
Regions Covered |
North America, Europe, Asia Pacific, South America, and Middle East & Africa |
|
Countries Covered |
US, Canada, Mexico, Germany, UK, Italy, France, Spain, Japan, China, India, Australia, New Zealand, South Korea, Rest of Asia Pacific, South America, Argentina, Brazil, Chile, Middle East & Africa, Saudi Arabia, UAE, Qatar, and South Africa |
|
Largest Market |
Asia Pacific |
|
Highest Growing Market |
North America |
According to Stratistics MRC, the Global Influenza Diagnostic Tests Market is accounted for $1.1 billion in 2023 and is expected to reach $1.7 billion by 2030 growing at a CAGR of 5.6% during the forecast period. Influenza diagnostic tests are medical procedures designed to identify the presence of influenza viruses in individuals experiencing respiratory symptoms. Emerging technologies, such as loop-mediated isothermal amplification (LAMP) and nucleic acid sequence-based amplification (NASBA), offer faster and simpler alternatives. Rapid and accurate diagnosis is crucial for prompt treatment and effective public health measures that amplify and detect viruses, providing accurate results.
According to estimates by the Centers for Disease Control and Prevention, there were 9 million flu cases, 4 million influenza-related medical appointments, 100,000 flu-related hospitalizations, and 5,000 flu-related fatalities during the 2021–2022 seasons in the U.S. Similar to recent seasons preceding the COVID-19 outbreak, 83% of fatalities were caused by older people.
Market Dynamics:
Driver:
Increased awareness and education
Health literacy programs educate individuals on recognizing influenza symptoms, understanding the potential severity of the disease, and appreciating the role of diagnostic tests in effective treatment and prevention. Public health campaigns, educational initiatives, and communication efforts contribute to raising awareness among healthcare professionals and the general public. Furthermore, healthcare providers are better equipped to recognize and respond to influenza cases, which are boosting this market expansion.
Restraint:
High cost
The high costs associated with influenza diagnostic tests can deter healthcare providers and patients from utilizing them, particularly in resource-limited settings or regions with constrained healthcare budgets. This can result in underdiagnosis, delayed treatment, and compromised disease management. In addition, there may be additional expenses related to test administration, such as professional fees and specimen collection supplies, hampering market growth.
Opportunity:
Advancements in diagnostic technologies
Molecular diagnostic technologies, notably reverse transcription-polymerase chain reaction (RT-PCR), have seen remarkable progress, offering heightened sensitivity and specificity in detecting influenza viruses. These advancements enable rapid and accurate identification of viral strains, aiding healthcare professionals in timely decision-making and patient management. Moreover, immunoassays and nucleic acid amplification techniques continue to evolve, contributing to a diverse array of diagnostic options, thereby driving this market size.
Threat:
Lack of awareness
Healthcare providers may not be familiar with the advantages, limitations, and proper interpretation of the results associated with each test. This can lead to underutilization or inappropriate utilization of diagnostic tests, potentially resulting in delayed diagnosis and suboptimal patient management. Moreover, many individuals may not be aware of its importance, which contributes to a lower demand for testing services, leading to missed opportunities for early detection and effective disease control and impeding this market.
Covid-19 Impact
The COVID-19 pandemic has had a negative impact on the influenza diagnostic test market in several ways. Healthcare systems worldwide have prioritized COVID-19 diagnostics, leading to a decrease in the overall demand for influenza diagnostic tests. The symptoms of COVID-19 and influenza often overlap, making it challenging to differentiate between the two based solely on clinical presentations. Further, the pandemic has also influenced healthcare spending priorities, potentially affecting investments in diagnostic technologies for influenza and hindering the market’s expansion.
The molecular diagnostic assay segment is expected to be the largest during the forecast period
The molecular diagnostic assay segment is estimated to hold the largest share due to a category of tests that utilize molecular biology techniques to detect and identify influenza viruses. These tests are known for their high sensitivity, specificity, and capacity to distinguish between different influenza subtypes. Moreover, they contribute to a more comprehensive understanding of the genetic characteristics of circulating influenza viruses using prominent methods like reverse transcription-polymerase chain reaction (RT-PCR), boosting this segment’s growth.
The research laboratories segment is expected to have the highest CAGR during the forecast period
The research laboratories segment is anticipated to have highest CAGR during the forecast period, due to they play a pivotal role in advancing the field of influenza diagnostics by conducting studies that aim to enhance the sensitivity, specificity, and efficiency of existing tests. They engage in the development of novel diagnostic assays, such as advanced molecular techniques, point-of-care testing devices, and serological assays. Furthermore, Research laboratories also play a key role in validating new diagnostic technologies providing essential data to guide healthcare practitioners propelling this segment’s expansion.
Region with largest share:
Asia Pacific commanded the largest market share during the extrapolated period owing to a growing adoption of advanced diagnostic technologies, including molecular assays like PCR, as they offer higher sensitivity and specificity. This region is home to some of the major key markets, such as BioConcept, Abbott, Quidel Corporation, and Thermo Fisher Scientific Inc. In addition, the region's high population density and interconnectedness emphasize the need for effective influenza surveillance and control measures that drive the market in this region.
Region with highest CAGR:
North America is expected to witness highest CAGR over the projection period, owing to a well-established healthcare infrastructure and a high level of awareness regarding infectious diseases. There is a widespread adoption of molecular diagnostic assays such as RT-PCR, which offer high sensitivity and accuracy in influenza detection. Moreover, continuous innovation in diagnostic technologies, ensuring that they align with emerging viral strains and global health challenges, thereby boosts this region’s growth.
Key players in the market
Some of the key players in the Influenza Diagnostic Tests Market include Becton, Dickinson and Company , Coris BioConcept, Meridian Bioscience Inc., Abbott, Sekisui Diagnostics, F. Hoffmann-La Roche Ltd, Hologic Inc., DiaSorin SpA, Quidel Corporation and Thermo Fischer Scientific Inc.
Key Developments:
In September 2023, Navigate BioPharma Services, Inc., announced a strategic collaboration with BD (Becton, Dickinson and Company), to explore opportunities to develop and commercialize flow cytometry-based companion diagnostics and tools for clinical decisions.
In September 2023, Abbott announced it has completed the acquisition of Bigfoot Biomedical, a leader in developing smart insulin management systems for people with diabetes.
In July 2023, Thermo Fisher Scientific Inc. announced it has entered into a definitive agreement to acquire CorEvitas, LLC (“CorEvitas”), for approved medical treatments and therapies, from Audax Private Equity (“Audax”).
In May 2023, Thermo Fisher, has signed a Memorandum of Understanding (""MOU"") with the National Research and Innovation Agency of Indonesia (BRIN, Badan Riset dan Inovasi Nasional) to enable and enhance the country's national research and innovation infrastructure and capability.
Test Types Covered:
• Molecular Diagnostic Assay
• Traditional Diagnostic Test
• Other Test Types
End Users Covered:
• Research Laboratories
• Hospitals
• Diagnostics Centers
• Other End Users
Regions Covered:
• North America
o US
o Canada
o Mexico
• Europe
o Germany
o UK
o Italy
o France
o Spain
o Rest of Europe
• Asia Pacific
o Japan
o China
o India
o Australia
o New Zealand
o South Korea
o Rest of Asia Pacific
• South America
o Argentina
o Brazil
o Chile
o Rest of South America
• Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Rest of Middle East & Africa
What our report offers:
- Market share assessments for the regional and country-level segments
- Strategic recommendations for the new entrants
- Covers Market data for the years 2021, 2022, 2023, 2026, and 2030
- Market Trends (Drivers, Constraints, Opportunities, Threats, Challenges, Investment Opportunities, and recommendations)
- Strategic recommendations in key business segments based on the market estimations
- Competitive landscaping mapping the key common trends
- Company profiling with detailed strategies, financials, and recent developments
- Supply chain trends mapping the latest technological advancements
Free Customization Offerings:
All the customers of this report will be entitled to receive one of the following free customization options:
• Company Profiling
o Comprehensive profiling of additional market players (up to 3)
o SWOT Analysis of key players (up to 3)
• Regional Segmentation
o Market estimations, Forecasts and CAGR of any prominent country as per the client's interest (Note: Depends on feasibility check)
• Competitive Benchmarking
Benchmarking of key players based on product portfolio, geographical presence, and strategic alliances
Table of Contents
1 Executive Summary
2 Preface
2.1 Abstract
2.2 Stake Holders
2.3 Research Scope
2.4 Research Methodology
2.4.1 Data Mining
2.4.2 Data Analysis
2.4.3 Data Validation
2.4.4 Research Approach
2.5 Research Sources
2.5.1 Primary Research Sources
2.5.2 Secondary Research Sources
2.5.3 Assumptions
3 Market Trend Analysis
3.1 Introduction
3.2 Drivers
3.3 Restraints
3.4 Opportunities
3.5 Threats
3.6 End User Analysis
3.7 Emerging Markets
3.8 Impact of Covid-19
4 Porters Five Force Analysis
4.1 Bargaining power of suppliers
4.2 Bargaining power of buyers
4.3 Threat of substitutes
4.4 Threat of new entrants
4.5 Competitive rivalry
5 Global Influenza Diagnostic Tests Market, By Test Type
5.1 Introduction
5.2 Molecular Diagnostic Assay
5.2.1 Simple Amplification-Based Assay (SAMBA)
5.2.2 RT-PCR
5.2.3 Nucleic Acid Sequence-Based Amplification Test (NASBAT)
5.2.4 Loop-Mediated Isothermal Amplification-Based Assay (LAMP)
5.2.5 Other Molecular Diagnostic Assays
5.3 Traditional Diagnostic Test
5.3.1 Viral Culture
5.3.2 Rapid Influenza Diagnostic Test (RIDT)
5.3.3 Serological Assay
5.3.4 Direct Fluorescent Antibody Test (DFAT)
5.4 Other Test Types
6 Global Influenza Diagnostic Tests Market, By End User
6.1 Introduction
6.2 Research Laboratories
6.3 Hospitals
6.4 Diagnostics Centers
6.5 Other End Users
7 Global Influenza Diagnostic Tests Market, By Geography
7.1 Introduction
7.2 North America
7.2.1 US
7.2.2 Canada
7.2.3 Mexico
7.3 Europe
7.3.1 Germany
7.3.2 UK
7.3.3 Italy
7.3.4 France
7.3.5 Spain
7.3.6 Rest of Europe
7.4 Asia Pacific
7.4.1 Japan
7.4.2 China
7.4.3 India
7.4.4 Australia
7.4.5 New Zealand
7.4.6 South Korea
7.4.7 Rest of Asia Pacific
7.5 South America
7.5.1 Argentina
7.5.2 Brazil
7.5.3 Chile
7.5.4 Rest of South America
7.6 Middle East & Africa
7.6.1 Saudi Arabia
7.6.2 UAE
7.6.3 Qatar
7.6.4 South Africa
7.6.5 Rest of Middle East & Africa
8 Key Developments
8.1 Agreements, Partnerships, Collaborations and Joint Ventures
8.2 Acquisitions & Mergers
8.3 New Product Launch
8.4 Expansions
8.5 Other Key Strategies
9 Company Profiling
9.1 Becton, Dickinson and Company
9.2 Coris BioConcept
9.3 Meridian Bioscience Inc.
9.4 Abbott
9.5 Sekisui Diagnostics
9.6 F. Hoffmann-La Roche Ltd
9.7 Hologic Inc.
9.8 DiaSorin SpA
9.9 Quidel Corporation
9.10 Thermo Fischer Scientific Inc.
List of Tables
1 Global Influenza Diagnostic Tests Market Outlook, By Region (2021-2030) ($MN)
2 Global Influenza Diagnostic Tests Market Outlook, By Test Type (2021-2030) ($MN)
3 Global Influenza Diagnostic Tests Market Outlook, By Molecular Diagnostic Assay (2021-2030) ($MN)
4 Global Influenza Diagnostic Tests Market Outlook, By Simple Amplification-Based Assay (SAMBA) (2021-2030) ($MN)
5 Global Influenza Diagnostic Tests Market Outlook, By RT-PCR (2021-2030) ($MN)
6 Global Influenza Diagnostic Tests Market Outlook, By Nucleic Acid Sequence-Based Amplification Test (NASBAT) (2021-2030) ($MN)
7 Global Influenza Diagnostic Tests Market Outlook, By Loop-Mediated Isothermal Amplification-Based Assay (LAMP) (2021-2030) ($MN)
8 Global Influenza Diagnostic Tests Market Outlook, By Other Molecular Diagnostic Assays (2021-2030) ($MN)
9 Global Influenza Diagnostic Tests Market Outlook, By Traditional Diagnostic Test (2021-2030) ($MN)
10 Global Influenza Diagnostic Tests Market Outlook, By Viral Culture (2021-2030) ($MN)
7 Global Influenza Diagnostic Tests Market Outlook, By Rapid Influenza Diagnostic Test (RIDT) (2021-2030) ($MN)
8 Global Influenza Diagnostic Tests Market Outlook, By Serological Assay (2021-2030) ($MN)
9 Global Influenza Diagnostic Tests Market Outlook, By Direct Fluorescent Antibody Test (DFAT) (2021-2030) ($MN)
14 Global Influenza Diagnostic Tests Market Outlook, By Other Test Types (2021-2030) ($MN)
15 Global Influenza Diagnostic Tests Market Outlook, By End User (2021-2030) ($MN)
16 Global Influenza Diagnostic Tests Market Outlook, By Research Laboratories (2021-2030) ($MN)
17 Global Influenza Diagnostic Tests Market Outlook, By Hospitals (2021-2030) ($MN)
18 Global Influenza Diagnostic Tests Market Outlook, By Diagnostics Centers (2021-2030) ($MN)
19 Global Influenza Diagnostic Tests Market Outlook, By Other End Users (2021-2030) ($MN)
20 North America Influenza Diagnostic Tests Market Outlook, By Country (2021-2030) ($MN)
21 North America Influenza Diagnostic Tests Market Outlook, By Test Type (2021-2030) ($MN)
22 North America Influenza Diagnostic Tests Market Outlook, By Molecular Diagnostic Assay (2021-2030) ($MN)
23 North America Influenza Diagnostic Tests Market Outlook, By Simple Amplification-Based Assay (SAMBA) (2021-2030) ($MN)
24 North America Influenza Diagnostic Tests Market Outlook, By RT-PCR (2021-2030) ($MN)
25 North America Influenza Diagnostic Tests Market Outlook, By Nucleic Acid Sequence-Based Amplification Test (NASBAT) (2021-2030) ($MN)
26 North America Influenza Diagnostic Tests Market Outlook, By Loop-Mediated Isothermal Amplification-Based Assay (LAMP) (2021-2030) ($MN)
27 North America Influenza Diagnostic Tests Market Outlook, By Other Molecular Diagnostic Assays (2021-2030) ($MN)
28 North America Influenza Diagnostic Tests Market Outlook, By Traditional Diagnostic Test (2021-2030) ($MN)
29 North America Influenza Diagnostic Tests Market Outlook, By Viral Culture (2021-2030) ($MN)
30 North America Influenza Diagnostic Tests Market Outlook, By Rapid Influenza Diagnostic Test (RIDT) (2021-2030) ($MN)
31 North America Influenza Diagnostic Tests Market Outlook, By Serological Assay (2021-2030) ($MN)
32 North America Influenza Diagnostic Tests Market Outlook, By Direct Fluorescent Antibody Test (DFAT) (2021-2030) ($MN)
33 North America Influenza Diagnostic Tests Market Outlook, By Other Test Types (2021-2030) ($MN)
34 North America Influenza Diagnostic Tests Market Outlook, By End User (2021-2030) ($MN)
35 North America Influenza Diagnostic Tests Market Outlook, By Research Laboratories (2021-2030) ($MN)
36 North America Influenza Diagnostic Tests Market Outlook, By Hospitals (2021-2030) ($MN)
37 North America Influenza Diagnostic Tests Market Outlook, By Diagnostics Centers (2021-2030) ($MN)
38 North America Influenza Diagnostic Tests Market Outlook, By Other End Users (2021-2030) ($MN)
39 Europe Influenza Diagnostic Tests Market Outlook, By Country (2021-2030) ($MN)
40 Europe Influenza Diagnostic Tests Market Outlook, By Test Type (2021-2030) ($MN)
41 Europe Influenza Diagnostic Tests Market Outlook, By Molecular Diagnostic Assay (2021-2030) ($MN)
42 Europe Influenza Diagnostic Tests Market Outlook, By Simple Amplification-Based Assay (SAMBA) (2021-2030) ($MN)
43 Europe Influenza Diagnostic Tests Market Outlook, By RT-PCR (2021-2030) ($MN)
44 Europe Influenza Diagnostic Tests Market Outlook, By Nucleic Acid Sequence-Based Amplification Test (NASBAT) (2021-2030) ($MN)
45 Europe Influenza Diagnostic Tests Market Outlook, By Loop-Mediated Isothermal Amplification-Based Assay (LAMP) (2021-2030) ($MN)
46 Europe Influenza Diagnostic Tests Market Outlook, By Other Molecular Diagnostic Assays (2021-2030) ($MN)
47 Europe Influenza Diagnostic Tests Market Outlook, By Traditional Diagnostic Test (2021-2030) ($MN)
48 Europe Influenza Diagnostic Tests Market Outlook, By Viral Culture (2021-2030) ($MN)
49 Europe Influenza Diagnostic Tests Market Outlook, By Rapid Influenza Diagnostic Test (RIDT) (2021-2030) ($MN)
50 Europe Influenza Diagnostic Tests Market Outlook, By Serological Assay (2021-2030) ($MN)
51 Europe Influenza Diagnostic Tests Market Outlook, By Direct Fluorescent Antibody Test (DFAT) (2021-2030) ($MN)
52 Europe Influenza Diagnostic Tests Market Outlook, By Other Test Types (2021-2030) ($MN)
53 Europe Influenza Diagnostic Tests Market Outlook, By End User (2021-2030) ($MN)
54 Europe Influenza Diagnostic Tests Market Outlook, By Research Laboratories (2021-2030) ($MN)
55 Europe Influenza Diagnostic Tests Market Outlook, By Hospitals (2021-2030) ($MN)
56 Europe Influenza Diagnostic Tests Market Outlook, By Diagnostics Centers (2021-2030) ($MN)
57 Europe Influenza Diagnostic Tests Market Outlook, By Other End Users (2021-2030) ($MN)
58 Asia Pacific Influenza Diagnostic Tests Market Outlook, By Country (2021-2030) ($MN)
59 Asia Pacific Influenza Diagnostic Tests Market Outlook, By Test Type (2021-2030) ($MN)
60 Asia Pacific Influenza Diagnostic Tests Market Outlook, By Molecular Diagnostic Assay (2021-2030) ($MN)
61 Asia Pacific Influenza Diagnostic Tests Market Outlook, By Simple Amplification-Based Assay (SAMBA) (2021-2030) ($MN)
62 Asia Pacific Influenza Diagnostic Tests Market Outlook, By RT-PCR (2021-2030) ($MN)
63 Asia Pacific Influenza Diagnostic Tests Market Outlook, By Nucleic Acid Sequence-Based Amplification Test (NASBAT) (2021-2030) ($MN)
64 Asia Pacific Influenza Diagnostic Tests Market Outlook, By Loop-Mediated Isothermal Amplification-Based Assay (LAMP) (2021-2030) ($MN)
65 Asia Pacific Influenza Diagnostic Tests Market Outlook, By Other Molecular Diagnostic Assays (2021-2030) ($MN)
66 Asia Pacific Influenza Diagnostic Tests Market Outlook, By Traditional Diagnostic Test (2021-2030) ($MN)
67 Asia Pacific Influenza Diagnostic Tests Market Outlook, By Viral Culture (2021-2030) ($MN)
68 Asia Pacific Influenza Diagnostic Tests Market Outlook, By Rapid Influenza Diagnostic Test (RIDT) (2021-2030) ($MN)
69 Asia Pacific Influenza Diagnostic Tests Market Outlook, By Serological Assay (2021-2030) ($MN)
70 Asia Pacific Influenza Diagnostic Tests Market Outlook, By Direct Fluorescent Antibody Test (DFAT) (2021-2030) ($MN)
71 Asia Pacific Influenza Diagnostic Tests Market Outlook, By Other Test Types (2021-2030) ($MN)
72 Asia Pacific Influenza Diagnostic Tests Market Outlook, By End User (2021-2030) ($MN)
73 Asia Pacific Influenza Diagnostic Tests Market Outlook, By Research Laboratories (2021-2030) ($MN)
74 Asia Pacific Influenza Diagnostic Tests Market Outlook, By Hospitals (2021-2030) ($MN)
75 Asia Pacific Influenza Diagnostic Tests Market Outlook, By Diagnostics Centers (2021-2030) ($MN)
76 Asia Pacific Influenza Diagnostic Tests Market Outlook, By Other End Users (2021-2030) ($MN)
77 South America Influenza Diagnostic Tests Market Outlook, By Country (2021-2030) ($MN)
78 South America Influenza Diagnostic Tests Market Outlook, By Test Type (2021-2030) ($MN)
79 South America Influenza Diagnostic Tests Market Outlook, By Molecular Diagnostic Assay (2021-2030) ($MN)
80 South America Influenza Diagnostic Tests Market Outlook, By Simple Amplification-Based Assay (SAMBA) (2021-2030) ($MN)
81 South America Influenza Diagnostic Tests Market Outlook, By RT-PCR (2021-2030) ($MN)
82 South America Influenza Diagnostic Tests Market Outlook, By Nucleic Acid Sequence-Based Amplification Test (NASBAT) (2021-2030) ($MN)
83 South America Influenza Diagnostic Tests Market Outlook, By Loop-Mediated Isothermal Amplification-Based Assay (LAMP) (2021-2030) ($MN)
84 South America Influenza Diagnostic Tests Market Outlook, By Other Molecular Diagnostic Assays (2021-2030) ($MN)
85 South America Influenza Diagnostic Tests Market Outlook, By Traditional Diagnostic Test (2021-2030) ($MN)
86 South America Influenza Diagnostic Tests Market Outlook, By Viral Culture (2021-2030) ($MN)
87 South America Influenza Diagnostic Tests Market Outlook, By Rapid Influenza Diagnostic Test (RIDT) (2021-2030) ($MN)
88 South America Influenza Diagnostic Tests Market Outlook, By Serological Assay (2021-2030) ($MN)
89 South America Influenza Diagnostic Tests Market Outlook, By Direct Fluorescent Antibody Test (DFAT) (2021-2030) ($MN)
90 South America Influenza Diagnostic Tests Market Outlook, By Other Test Types (2021-2030) ($MN)
91 South America Influenza Diagnostic Tests Market Outlook, By End User (2021-2030) ($MN)
92 South America Influenza Diagnostic Tests Market Outlook, By Research Laboratories (2021-2030) ($MN)
93 South America Influenza Diagnostic Tests Market Outlook, By Hospitals (2021-2030) ($MN)
94 South America Influenza Diagnostic Tests Market Outlook, By Diagnostics Centers (2021-2030) ($MN)
95 South America Influenza Diagnostic Tests Market Outlook, By Other End Users (2021-2030) ($MN)
96 Middle East & Africa Influenza Diagnostic Tests Market Outlook, By Country (2021-2030) ($MN)
97 Middle East & Africa Influenza Diagnostic Tests Market Outlook, By Test Type (2021-2030) ($MN)
98 Middle East & Africa Influenza Diagnostic Tests Market Outlook, By Molecular Diagnostic Assay (2021-2030) ($MN)
99 Middle East & Africa Influenza Diagnostic Tests Market Outlook, By Simple Amplification-Based Assay (SAMBA) (2021-2030) ($MN)
100 Middle East & Africa Influenza Diagnostic Tests Market Outlook, By RT-PCR (2021-2030) ($MN)
101 Middle East & Africa Influenza Diagnostic Tests Market Outlook, By Nucleic Acid Sequence-Based Amplification Test (NASBAT) (2021-2030) ($MN)
102 Middle East & Africa Influenza Diagnostic Tests Market Outlook, By Loop-Mediated Isothermal Amplification-Based Assay (LAMP) (2021-2030) ($MN)
103 Middle East & Africa Influenza Diagnostic Tests Market Outlook, By Other Molecular Diagnostic Assays (2021-2030) ($MN)
104 Middle East & Africa Influenza Diagnostic Tests Market Outlook, By Traditional Diagnostic Test (2021-2030) ($MN)
105 Middle East & Africa Influenza Diagnostic Tests Market Outlook, By Viral Culture (2021-2030) ($MN)
106 Middle East & Africa Influenza Diagnostic Tests Market Outlook, By Rapid Influenza Diagnostic Test (RIDT) (2021-2030) ($MN)
107 Middle East & Africa Influenza Diagnostic Tests Market Outlook, By Serological Assay (2021-2030) ($MN)
108 Middle East & Africa Influenza Diagnostic Tests Market Outlook, By Direct Fluorescent Antibody Test (DFAT) (2021-2030) ($MN)
109 Middle East & Africa Influenza Diagnostic Tests Market Outlook, By Other Test Types (2021-2030) ($MN)
110 Middle East & Africa Influenza Diagnostic Tests Market Outlook, By End User (2021-2030) ($MN)
111 Middle East & Africa Influenza Diagnostic Tests Market Outlook, By Research Laboratories (2021-2030) ($MN)
112 Middle East & Africa Influenza Diagnostic Tests Market Outlook, By Hospitals (2021-2030) ($MN)
113 Middle East & Africa Influenza Diagnostic Tests Market Outlook, By Diagnostics Centers (2021-2030) ($MN)
114 Middle East & Africa Influenza Diagnostic Tests Market Outlook, By Other End Users (2021-2030) ($MN)
List of Figures
RESEARCH METHODOLOGY
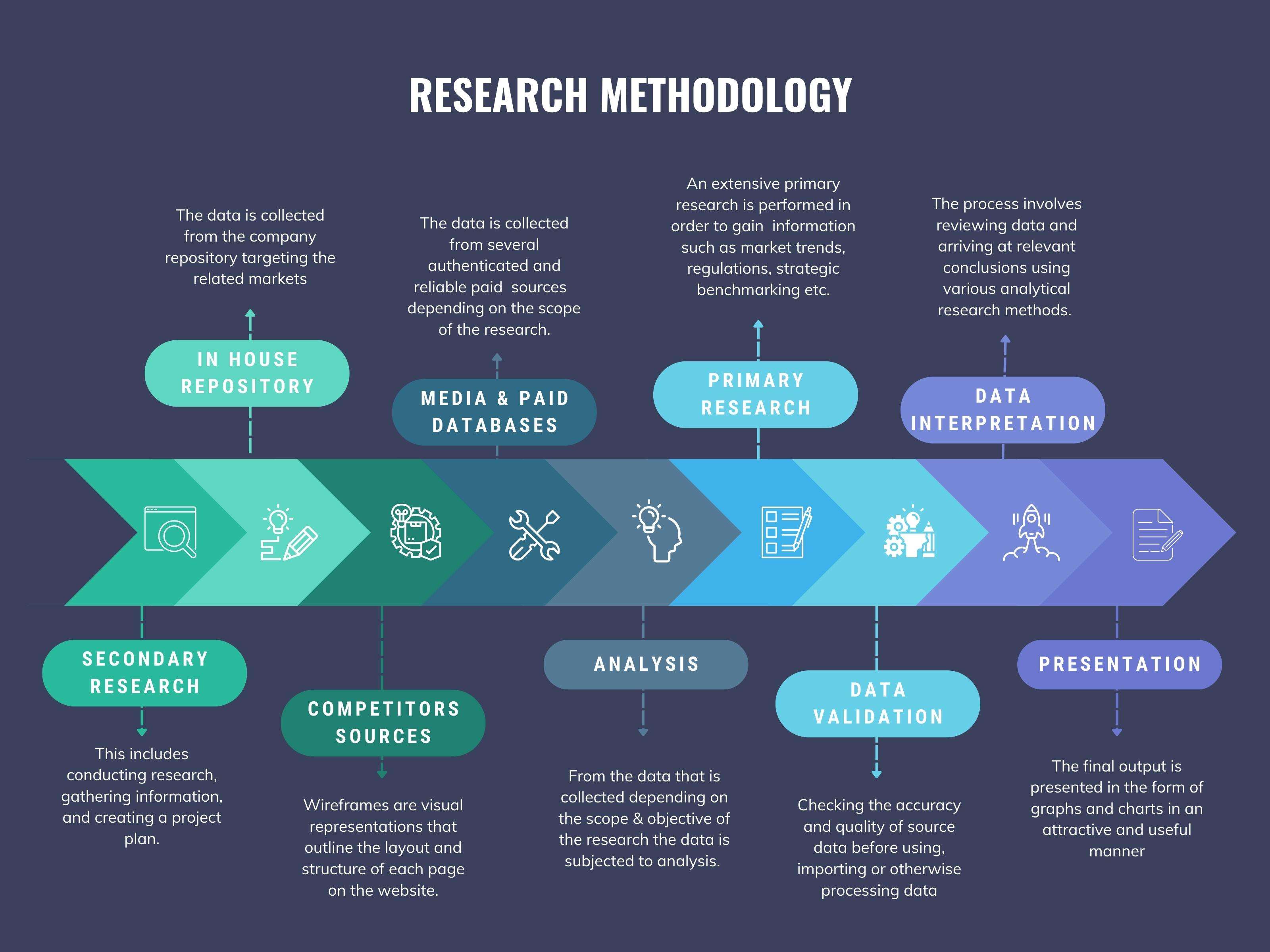
We at ‘Stratistics’ opt for an extensive research approach which involves data mining, data validation, and data analysis. The various research sources include in-house repository, secondary research, competitor’s sources, social media research, client internal data, and primary research.
Our team of analysts prefers the most reliable and authenticated data sources in order to perform the comprehensive literature search. With access to most of the authenticated data bases our team highly considers the best mix of information through various sources to obtain extensive and accurate analysis.
Each report takes an average time of a month and a team of 4 industry analysts. The time may vary depending on the scope and data availability of the desired market report. The various parameters used in the market assessment are standardized in order to enhance the data accuracy.
Data Mining
The data is collected from several authenticated, reliable, paid and unpaid sources and is filtered depending on the scope & objective of the research. Our reports repository acts as an added advantage in this procedure. Data gathering from the raw material suppliers, distributors and the manufacturers is performed on a regular basis, this helps in the comprehensive understanding of the products value chain. Apart from the above mentioned sources the data is also collected from the industry consultants to ensure the objective of the study is in the right direction.
Market trends such as technological advancements, regulatory affairs, market dynamics (Drivers, Restraints, Opportunities and Challenges) are obtained from scientific journals, market related national & international associations and organizations.
Data Analysis
From the data that is collected depending on the scope & objective of the research the data is subjected for the analysis. The critical steps that we follow for the data analysis include:
- Product Lifecycle Analysis
- Competitor analysis
- Risk analysis
- Porters Analysis
- PESTEL Analysis
- SWOT Analysis
The data engineering is performed by the core industry experts considering both the Marketing Mix Modeling and the Demand Forecasting. The marketing mix modeling makes use of multiple-regression techniques to predict the optimal mix of marketing variables. Regression factor is based on a number of variables and how they relate to an outcome such as sales or profits.
Data Validation
The data validation is performed by the exhaustive primary research from the expert interviews. This includes telephonic interviews, focus groups, face to face interviews, and questionnaires to validate our research from all aspects. The industry experts we approach come from the leading firms, involved in the supply chain ranging from the suppliers, distributors to the manufacturers and consumers so as to ensure an unbiased analysis.
We are in touch with more than 15,000 industry experts with the right mix of consultants, CEO's, presidents, vice presidents, managers, experts from both supply side and demand side, executives and so on.
The data validation involves the primary research from the industry experts belonging to:
- Leading Companies
- Suppliers & Distributors
- Manufacturers
- Consumers
- Industry/Strategic Consultants
Apart from the data validation the primary research also helps in performing the fill gap research, i.e. providing solutions for the unmet needs of the research which helps in enhancing the reports quality.
For more details about research methodology, kindly write to us at info@strategymrc.com
Frequently Asked Questions
In case of any queries regarding this report, you can contact the customer service by filing the “Inquiry Before Buy” form available on the right hand side. You may also contact us through email: info@strategymrc.com or phone: +1-301-202-5929
Yes, the samples are available for all the published reports. You can request them by filling the “Request Sample” option available in this page.
Yes, you can request a sample with your specific requirements. All the customized samples will be provided as per the requirement with the real data masked.
All our reports are available in Digital PDF format. In case if you require them in any other formats, such as PPT, Excel etc you can submit a request through “Inquiry Before Buy” form available on the right hand side. You may also contact us through email: info@strategymrc.com or phone: +1-301-202-5929
We offer a free 15% customization with every purchase. This requirement can be fulfilled for both pre and post sale. You may send your customization requirements through email at info@strategymrc.com or call us on +1-301-202-5929.
We have 3 different licensing options available in electronic format.
- Single User Licence: Allows one person, typically the buyer, to have access to the ordered product. The ordered product cannot be distributed to anyone else.
- 2-5 User Licence: Allows the ordered product to be shared among a maximum of 5 people within your organisation.
- Corporate License: Allows the product to be shared among all employees of your organisation regardless of their geographical location.
All our reports are typically be emailed to you as an attachment.
To order any available report you need to register on our website. The payment can be made either through CCAvenue or PayPal payments gateways which accept all international cards.
We extend our support to 6 months post sale. A post sale customization is also provided to cover your unmet needs in the report.
Request Customization
We provide a free 15% customization on every purchase. This requirement can be fulfilled for both pre and post sale. You may send your customization requirements through email at info@strategymrc.com or call us on +1-301-202-5929.
Note: This customization is absolutely free until it falls under the 15% bracket. If your requirement exceeds this a feasibility check will be performed. Post that, a quote will be provided along with the timelines.
WHY CHOOSE US ?

Assured Quality
Best in class reports with high standard of research integrity

24X7 Research Support
Continuous support to ensure the best customer experience.

Free Customization
Adding more values to your product of interest.

Safe & Secure Access
Providing a secured environment for all online transactions.

Trusted by 600+ Brands
Serving the most reputed brands across the world.
