
Influenza Diagnostics Market
Influenza Diagnostics Market Forecasts to 2030 - Global Analysis By Product (Instruments, Kits & Reagents and Other Products), Test Type (Traditional Diagnostic Test, Molecular Diagnostic Assay, Serological Testing and Other Test Types), End User and By Geography

|
Years Covered |
2021-2030 |
|
Estimated Year Value (2023) |
US $2.32 BN |
|
Projected Year Value (2030) |
US $4.06 BN |
|
CAGR (2023 - 2030) |
8.3% |
|
Regions Covered |
North America, Europe, Asia Pacific, South America, and Middle East & Africa |
|
Countries Covered |
US, Canada, Mexico, Germany, UK, Italy, France, Spain, Japan, China, India, Australia, New Zealand, South Korea, Rest of Asia Pacific, South America, Argentina, Brazil, Chile, Middle East & Africa, Saudi Arabia, UAE, Qatar, and South Africa |
|
Largest Market |
Asia Pacific |
|
Highest Growing Market |
North America |
According to Stratistics MRC, the Global Influenza Diagnostics Market is accounted for $2.32 billion in 2023 and is expected to reach $4.06 billion by 2030 growing at a CAGR of 8.3% during the forecast period. Influenza diagnostics involve methods to identify and confirm influenza virus infections. Rapid tests, such as antigen detection assays, provide quick results by detecting viral proteins in respiratory samples. Molecular tests, like polymerase chain reaction (PCR), offer high sensitivity and specificity by detecting viral genetic material. These methods aid in early diagnosis, guiding appropriate treatment and public health interventions. Timely and accurate influenza diagnostics enables effective patient management, outbreak control and vaccine development.
According to the World Health Organization report, in 2023, there were around a billion cases of seasonal influenza annually, including 3–5 million cases of severe illness. Moreover, influenza causes 290,000 to 650,000 respiratory deaths annually around the globe.

Market Dynamics:
Driver:
Rising prevalence of influenza
The increasing incidence of influenza cases worldwide underscores the critical need for efficient and rapid diagnostic tools. The market is witnessing a surge in demand for advanced diagnostic technologies capable of swift and accurate detection of influenza viruses. This heightened demand is steering research and development efforts towards innovative diagnostic solutions, fostering market expansion. As healthcare systems prioritize early detection and effective management of influenza, the influenza diagnostics market is poised to witness sustained growth, driven by the urgent global health imperative to mitigate the impact of influenza outbreaks.
Restraint:
Limited sensitivity & specificity
While influenza diagnostics play a crucial role in detecting influenza viruses, their accuracy in differentiating between subtypes and strains remains a concern. Rapid evolution and antigenic variability challenge the design of diagnostics, leading to false negatives or difficulty in distinguishing between influenza subtypes. False positives and negatives can lead to misdiagnoses, impacting patient care and public health efforts. Balancing the trade-off between sensitivity and specificity remains a complex task in influenza diagnostics, which hinder the market growth.
Opportunity:
Growing research & development activities
Ongoing advancements in diagnostic technologies, such as molecular and rapid testing methods, are enhancing the accuracy and efficiency of influenza detection. Increasing investments in R&D by key market players and government initiatives are driving innovation, leading to the development of more reliable and rapid diagnostic tools. This surge in R&D activities not only fosters technological breakthroughs but also opens avenues for novel diagnostic solutions, ultimately propelling the growth of the market. Also, the dynamic landscape of research in this field positions it as a crucial driver for market expansion.
Threat:
Competition from antiviral medications
As pharmaceutical advancements continue, antiviral drugs gain prominence as a primary intervention for influenza, potentially diminishing the demand for diagnostic tools. The availability of effective antiviral treatments may reduce the urgency for rapid and accurate diagnostic solutions, impacting the market's growth. The healthcare landscape's shift towards treatment-oriented approaches underscores the need for constant innovation in diagnostics to maintain relevance and competitiveness amid the evolving dynamics of influenza management.
Covid-19 Impact
The covid-19 pandemic has significantly influenced the influenza diagnostics market. Increased awareness of respiratory infections, heightened demand for diagnostic testing due to overlapping symptoms with covid, and a surge in healthcare infrastructure have positively impacted the market. Additionally, the focus on advanced diagnostic technologies and research in virology has driven innovation in influenza diagnostics. However, disruptions in the supply chain and healthcare services during the pandemic have presented challenges. Overall, the market has experienced a dynamic shift with both opportunities and obstacles arising from the epidemic impact.
The serological testing segment is expected to be the largest during the forecast period
The serological testing segment is estimated to have a lucrative growth. Serological testing plays a crucial role in Influenza Diagnostics by detecting antibodies in a patient's blood serum, aiding in the identification and monitoring of influenza infections. This method helps differentiate between current and past infections, allowing for a comprehensive understanding of an individual's immune response. Overall, the versatility of serological testing enhances diagnostic accuracy, informs preventative strategies, and strengthens the overall management of influenza infections.
The clinical laboratories segment is expected to have the highest CAGR during the forecast period
The clinical laboratories segment is anticipated to witness the highest CAGR growth during the forecast period. In clinical laboratories, influenza diagnostics play a pivotal role in patient care and public health. Rapid and accurate identification of influenza viruses aids in timely treatment decisions, reducing the severity and duration of illness. Diagnostic tests, including PCR and rapid antigen assays, enable early detection, helping healthcare professionals implement infection control measures to prevent further transmission. These tests enhance patient outcomes, support public health initiatives, and inform vaccination strategies, demonstrating their indispensable role in clinical laboratories for effective influenza management and control.
Region with largest share:
Asia Pacific is projected to hold the largest market share during the forecast period owing to the increased awareness, rising healthcare expenditure, and a surge in influenza cases. Advanced diagnostic technologies, such as PCR and rapid influenza detection tests, are gaining prominence in the region. Additionally, government initiatives coupled with the growing demand for point-of-care testing, are driving market expansion. Key players such as Roche Diagnostics, Abbott Laboratories and Thermo Fisher Scientific are focusing on strategic collaborations and product innovations to strengthen their market presence. The Asia Pacific Influenza Diagnostics Market is poised for continued growth, driven by a proactive approach to public health and the continual evolution of diagnostic technologies.
Region with highest CAGR:
North America is projected to have the highest CAGR over the forecast period, owing to the increasing prevalence of influenza and the constant need for accurate and timely diagnostic solutions. The region witnesses a robust demand for rapid and sensitive diagnostic tools, driving technological advancements. Key players in the market like Luminex Corporation, DiaSorin and Becton, Dickinson and Company (BD) focus on innovative techniques like PCR and immunoassays for efficient influenza detection. The market is influenced by factors such as government initiatives, rising awareness, and the need for point-of-care testing.
Key players in the market
Some of the key players profiled in the Influenza Diagnostics Market include Altona Diagnostics GmbH, ELITech Group, GenMark Diagnostics, F. Hoffmann-La Roche Ltd, Abbott Laboratories, Becton, Dickinson, and Company, Coris BioConcept, DiaSorin SpA, Meridian Bioscience Inc., Quidel Corporation, Sekisui Diagnostics, Thermo Fischer Scientific Inc., Hologic Inc., Mast Group, Siemens Healthineers AG, BioMerieux SA and Luminex Corporation.
Key Developments:
In September 2022, Siemens Healthineers released its new CE-marked FTD SARS-CoV-2/FluA/FluB/HRSV Assay, a PCR test, and the CLINITEST Rapid COVID-19 + Influenza Antigen Test. Validated on the company's VERSANT kPCR Molecular System, the kit combines Siemens Healthineers FTD SARS-CoV-23 and FTD Flu/HRSV Assays.
In February 2022, Roche announced the expansion of the COVID-19 PCR portfolio to the cobas® 5800 System, a recently launched molecular laboratory instrument, in countries accepting the CE mark. These include cobas SARS-CoV-2 Qualitative and cobas SARS-CoV-2 & Influenza A/B tests. These launches expand the Roche Diagnostics molecular portfolio offering by providing standardized performance and efficiencies across low, medium and high volume molecular laboratory testing needs.
Products Covered:
• Instruments
• Kits & Reagents
• Other Products
Test Types Covered:
• Traditional Diagnostic Test
• Molecular Diagnostic Assay
• Serological Testing
• Other Test Types
End Users Covered:
• Point-of-Care Testing
• Hospitals
• Clinical Laboratories
• Academic/Research Institutes
• Homecare Settings
• Other End Users
Regions Covered:
• North America
o US
o Canada
o Mexico
• Europe
o Germany
o UK
o Italy
o France
o Spain
o Rest of Europe
• Asia Pacific
o Japan
o China
o India
o Australia
o New Zealand
o South Korea
o Rest of Asia Pacific
• South America
o Argentina
o Brazil
o Chile
o Rest of South America
• Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Rest of Middle East & Africa
What our report offers:
- Market share assessments for the regional and country-level segments
- Strategic recommendations for the new entrants
- Covers Market data for the years 2021, 2022, 2023, 2026, and 2030
- Market Trends (Drivers, Constraints, Opportunities, Threats, Challenges, Investment Opportunities, and recommendations)
- Strategic recommendations in key business segments based on the market estimations
- Competitive landscaping mapping the key common trends
- Company profiling with detailed strategies, financials, and recent developments
- Supply chain trends mapping the latest technological advancements
Free Customization Offerings:
All the customers of this report will be entitled to receive one of the following free customization options:
• Company Profiling
o Comprehensive profiling of additional market players (up to 3)
o SWOT Analysis of key players (up to 3)
• Regional Segmentation
o Market estimations, Forecasts and CAGR of any prominent country as per the client's interest (Note: Depends on feasibility check)
• Competitive Benchmarking
o Benchmarking of key players based on product portfolio, geographical presence, and strategic alliances
Table of Contents
1 Executive Summary
2 Preface
2.1 Abstract
2.2 Stake Holders
2.3 Research Scope
2.4 Research Methodology
2.4.1 Data Mining
2.4.2 Data Analysis
2.4.3 Data Validation
2.4.4 Research Approach
2.5 Research Sources
2.5.1 Primary Research Sources
2.5.2 Secondary Research Sources
2.5.3 Assumptions
3 Market Trend Analysis
3.1 Introduction
3.2 Drivers
3.3 Restraints
3.4 Opportunities
3.5 Threats
3.6 Product Analysis
3.7 End User Analysis
3.8 Emerging Markets
3.9 Impact of Covid-19
4 Porters Five Force Analysis
4.1 Bargaining power of suppliers
4.2 Bargaining power of buyers
4.3 Threat of substitutes
4.4 Threat of new entrants
4.5 Competitive rivalry
5 Global Influenza Diagnostics Market, By Product
5.1 Introduction
5.2 Instruments
5.3 Kits & Reagents
5.4 Other Products
6 Global Influenza Diagnostics Market, By Test Type
6.1 Introduction
6.2 Traditional Diagnostic Test
6.2.1 Viral Culture
6.2.2 Direct Fluorescent Antibody Test (DFAT)
6.2.3 Rapid Influenza Diagnostic Test (RIDT)
6.3 Molecular Diagnostic Assay
6.3.1 Simple Amplification-based Assay (SAMBA)
6.3.2 Nucleic Acid Sequence-based Amplification Test (NASBAT)
6.3.3 Loop-mediated Isothermal Amplification-based Assay (LAMP)
6.3.4 Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
6.4 Serological Testing
6.4.1 Hemagglutination Inhibition (HI) Assays
6.4.2 Microneutralization Assays
6.5 Other Test Types
7 Global Influenza Diagnostics Market, By End User
7.1 Introduction
7.2 Point-of-Care Testing
7.3 Hospitals
7.4 Clinical Laboratories
7.5 Academic/Research Institutes
7.6 Homecare Settings
7.7 Other End Users
8 Global Influenza Diagnostics Market, By Geography
8.1 Introduction
8.2 North America
8.2.1 US
8.2.2 Canada
8.2.3 Mexico
8.3 Europe
8.3.1 Germany
8.3.2 UK
8.3.3 Italy
8.3.4 France
8.3.5 Spain
8.3.6 Rest of Europe
8.4 Asia Pacific
8.4.1 Japan
8.4.2 China
8.4.3 India
8.4.4 Australia
8.4.5 New Zealand
8.4.6 South Korea
8.4.7 Rest of Asia Pacific
8.5 South America
8.5.1 Argentina
8.5.2 Brazil
8.5.3 Chile
8.5.4 Rest of South America
8.6 Middle East & Africa
8.6.1 Saudi Arabia
8.6.2 UAE
8.6.3 Qatar
8.6.4 South Africa
8.6.5 Rest of Middle East & Africa
9 Key Developments
9.1 Agreements, Partnerships, Collaborations and Joint Ventures
9.2 Acquisitions & Mergers
9.3 New Product Launch
9.4 Expansions
9.5 Other Key Strategies
10 Company Profiling
10.1 Altona Diagnostics GmbH
10.2 ELITech Group
10.3 GenMark Diagnostics
10.4 F. Hoffmann-La Roche Ltd
10.5 Abbott Laboratories
10.6 Becton, Dickinson, and Company
10.7 Coris BioConcept
10.8 DiaSorin SpA
10.9 Meridian Bioscience Inc.
10.10 Quidel Corporation
10.11 Sekisui Diagnostics
10.12 Thermo Fischer Scientific Inc.
10.13 Hologic Inc.
10.14 Mast Group
10.15 Siemens Healthineers AG
10.16 BioMerieux SA
10.17 Luminex Corporation
List of Tables
1 Global Influenza Diagnostics Market Outlook, By Region (2021-2030) ($MN)
2 Global Influenza Diagnostics Market Outlook, By Product (2021-2030) ($MN)
3 Global Influenza Diagnostics Market Outlook, By Instruments (2021-2030) ($MN)
4 Global Influenza Diagnostics Market Outlook, By Kits & Reagents (2021-2030) ($MN)
5 Global Influenza Diagnostics Market Outlook, By Other Products (2021-2030) ($MN)
6 Global Influenza Diagnostics Market Outlook, By Test Type (2021-2030) ($MN)
7 Global Influenza Diagnostics Market Outlook, By Traditional Diagnostic Test (2021-2030) ($MN)
8 Global Influenza Diagnostics Market Outlook, By Viral Culture (2021-2030) ($MN)
9 Global Influenza Diagnostics Market Outlook, By Direct Fluorescent Antibody Test (DFAT) (2021-2030) ($MN)
10 Global Influenza Diagnostics Market Outlook, By Rapid Influenza Diagnostic Test (RIDT) (2021-2030) ($MN)
11 Global Influenza Diagnostics Market Outlook, By Molecular Diagnostic Assay (2021-2030) ($MN)
12 Global Influenza Diagnostics Market Outlook, By Simple Amplification-based Assay (SAMBA) (2021-2030) ($MN)
13 Global Influenza Diagnostics Market Outlook, By Nucleic Acid Sequence-based Amplification Test (NASBAT) (2021-2030) ($MN)
14 Global Influenza Diagnostics Market Outlook, By Loop-mediated Isothermal Amplification-based Assay (LAMP) (2021-2030) ($MN)
15 Global Influenza Diagnostics Market Outlook, By Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) (2021-2030) ($MN)
16 Global Influenza Diagnostics Market Outlook, By Serological Testing (2021-2030) ($MN)
17 Global Influenza Diagnostics Market Outlook, By Hemagglutination Inhibition (HI) Assays (2021-2030) ($MN)
18 Global Influenza Diagnostics Market Outlook, By Microneutralization Assays (2021-2030) ($MN)
19 Global Influenza Diagnostics Market Outlook, By Other Test Types (2021-2030) ($MN)
20 Global Influenza Diagnostics Market Outlook, By End User (2021-2030) ($MN)
21 Global Influenza Diagnostics Market Outlook, By Point-of-Care Testing (2021-2030) ($MN)
22 Global Influenza Diagnostics Market Outlook, By Hospitals (2021-2030) ($MN)
23 Global Influenza Diagnostics Market Outlook, By Clinical Laboratories (2021-2030) ($MN)
24 Global Influenza Diagnostics Market Outlook, By Academic/Research Institutes (2021-2030) ($MN)
25 Global Influenza Diagnostics Market Outlook, By Homecare Settings (2021-2030) ($MN)
26 Global Influenza Diagnostics Market Outlook, By Other End Users (2021-2030) ($MN)
Note: Tables for North America, Europe, APAC, South America, and Middle East & Africa Regions are also represented in the same manner as above.
List of Figures
RESEARCH METHODOLOGY
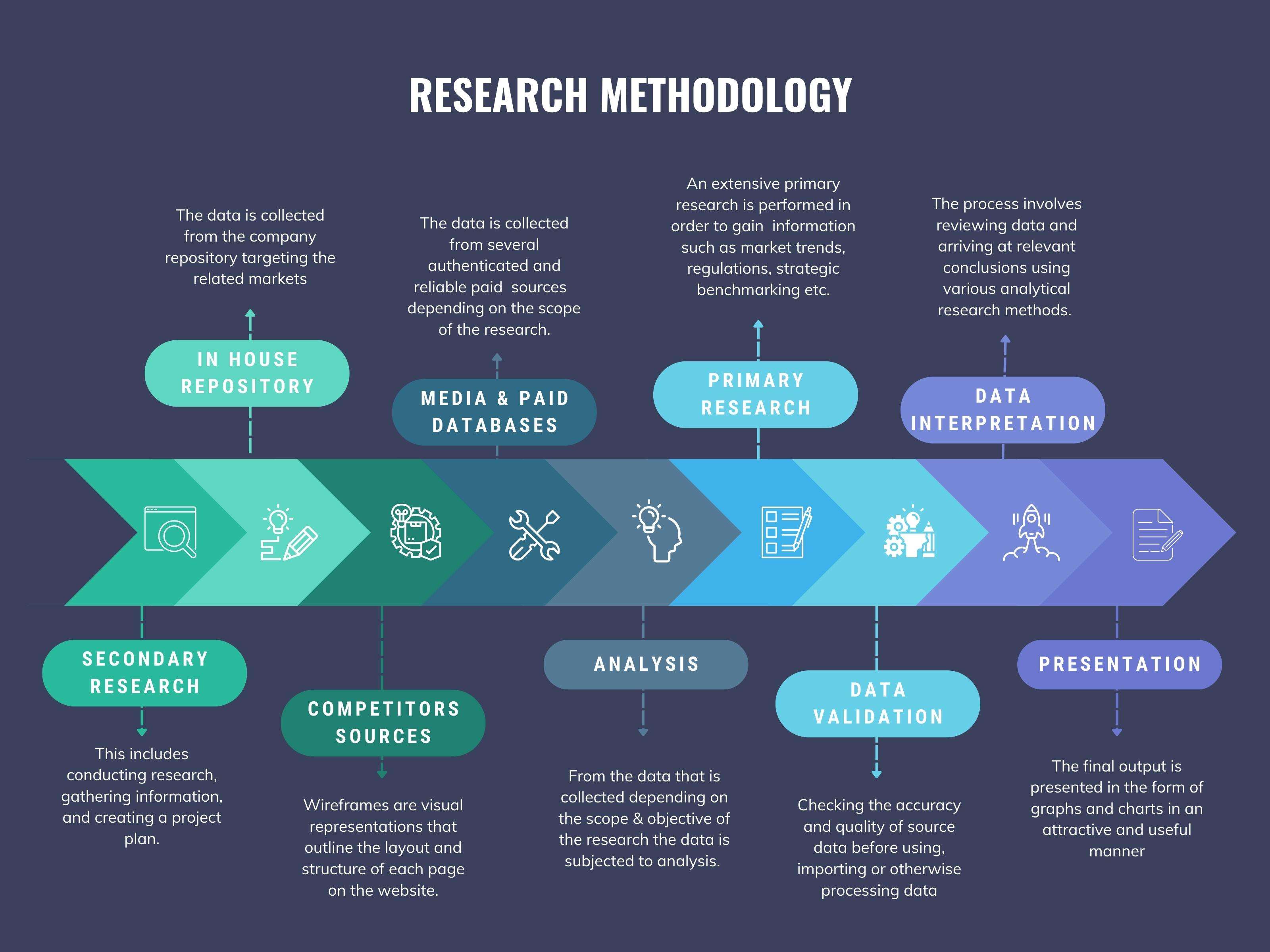
We at ‘Stratistics’ opt for an extensive research approach which involves data mining, data validation, and data analysis. The various research sources include in-house repository, secondary research, competitor’s sources, social media research, client internal data, and primary research.
Our team of analysts prefers the most reliable and authenticated data sources in order to perform the comprehensive literature search. With access to most of the authenticated data bases our team highly considers the best mix of information through various sources to obtain extensive and accurate analysis.
Each report takes an average time of a month and a team of 4 industry analysts. The time may vary depending on the scope and data availability of the desired market report. The various parameters used in the market assessment are standardized in order to enhance the data accuracy.
Data Mining
The data is collected from several authenticated, reliable, paid and unpaid sources and is filtered depending on the scope & objective of the research. Our reports repository acts as an added advantage in this procedure. Data gathering from the raw material suppliers, distributors and the manufacturers is performed on a regular basis, this helps in the comprehensive understanding of the products value chain. Apart from the above mentioned sources the data is also collected from the industry consultants to ensure the objective of the study is in the right direction.
Market trends such as technological advancements, regulatory affairs, market dynamics (Drivers, Restraints, Opportunities and Challenges) are obtained from scientific journals, market related national & international associations and organizations.
Data Analysis
From the data that is collected depending on the scope & objective of the research the data is subjected for the analysis. The critical steps that we follow for the data analysis include:
- Product Lifecycle Analysis
- Competitor analysis
- Risk analysis
- Porters Analysis
- PESTEL Analysis
- SWOT Analysis
The data engineering is performed by the core industry experts considering both the Marketing Mix Modeling and the Demand Forecasting. The marketing mix modeling makes use of multiple-regression techniques to predict the optimal mix of marketing variables. Regression factor is based on a number of variables and how they relate to an outcome such as sales or profits.
Data Validation
The data validation is performed by the exhaustive primary research from the expert interviews. This includes telephonic interviews, focus groups, face to face interviews, and questionnaires to validate our research from all aspects. The industry experts we approach come from the leading firms, involved in the supply chain ranging from the suppliers, distributors to the manufacturers and consumers so as to ensure an unbiased analysis.
We are in touch with more than 15,000 industry experts with the right mix of consultants, CEO's, presidents, vice presidents, managers, experts from both supply side and demand side, executives and so on.
The data validation involves the primary research from the industry experts belonging to:
- Leading Companies
- Suppliers & Distributors
- Manufacturers
- Consumers
- Industry/Strategic Consultants
Apart from the data validation the primary research also helps in performing the fill gap research, i.e. providing solutions for the unmet needs of the research which helps in enhancing the reports quality.
For more details about research methodology, kindly write to us at info@strategymrc.com
Frequently Asked Questions
In case of any queries regarding this report, you can contact the customer service by filing the “Inquiry Before Buy” form available on the right hand side. You may also contact us through email: info@strategymrc.com or phone: +1-301-202-5929
Yes, the samples are available for all the published reports. You can request them by filling the “Request Sample” option available in this page.
Yes, you can request a sample with your specific requirements. All the customized samples will be provided as per the requirement with the real data masked.
All our reports are available in Digital PDF format. In case if you require them in any other formats, such as PPT, Excel etc you can submit a request through “Inquiry Before Buy” form available on the right hand side. You may also contact us through email: info@strategymrc.com or phone: +1-301-202-5929
We offer a free 15% customization with every purchase. This requirement can be fulfilled for both pre and post sale. You may send your customization requirements through email at info@strategymrc.com or call us on +1-301-202-5929.
We have 3 different licensing options available in electronic format.
- Single User Licence: Allows one person, typically the buyer, to have access to the ordered product. The ordered product cannot be distributed to anyone else.
- 2-5 User Licence: Allows the ordered product to be shared among a maximum of 5 people within your organisation.
- Corporate License: Allows the product to be shared among all employees of your organisation regardless of their geographical location.
All our reports are typically be emailed to you as an attachment.
To order any available report you need to register on our website. The payment can be made either through CCAvenue or PayPal payments gateways which accept all international cards.
We extend our support to 6 months post sale. A post sale customization is also provided to cover your unmet needs in the report.
Request Customization
We provide a free 15% customization on every purchase. This requirement can be fulfilled for both pre and post sale. You may send your customization requirements through email at info@strategymrc.com or call us on +1-301-202-5929.
Note: This customization is absolutely free until it falls under the 15% bracket. If your requirement exceeds this a feasibility check will be performed. Post that, a quote will be provided along with the timelines.
WHY CHOOSE US ?

Assured Quality
Best in class reports with high standard of research integrity

24X7 Research Support
Continuous support to ensure the best customer experience.

Free Customization
Adding more values to your product of interest.

Safe & Secure Access
Providing a secured environment for all online transactions.

Trusted by 600+ Brands
Serving the most reputed brands across the world.
