
Active Pharmaceutical Ingredients Market
Active Pharmaceutical Ingredients Market Forecasts to 2030 - Global Analysis By Drug Type (Branded or Innovative Prescription Drugs, Generic Prescription Drugs, Over-the-counter (OTC) Drugs and Other Drug Types), Manufacturer Type (In House Manufacturing, Contract Manufacturing and Other Manufacturer Types), API Type, Application, End User and by Geography

|
Years Covered |
2021-2030 |
|
Estimated Year Value (2023) |
US $163.75 BN |
|
Projected Year Value (2030) |
US $323.19 BN |
|
CAGR (2023 - 2030) |
10.2% |
|
Regions Covered |
North America, Europe, Asia Pacific, South America, and Middle East & Africa |
|
Countries Covered |
US, Canada, Mexico, Germany, UK, Italy, France, Spain, Japan, China, India, Australia, New Zealand, South Korea, Rest of Asia Pacific, South America, Argentina, Brazil, Chile, Middle East & Africa, Saudi Arabia, UAE, Qatar, and South Africa |
|
Largest Market |
North America |
|
Highest Growing Market |
Europe |
According to Stratistics MRC, the Global Active Pharmaceutical Ingredients Market is accounted for $163.75 billion in 2023 and is expected to reach $323.19 billion by 2030 growing at a CAGR of 10.2% during the forecast period. The essential elements of pharmaceuticals that result in the desired therapeutic effect are known as active pharmaceutical ingredients, or APIs. These chemicals go through rigorous testing and are approved by the government before being used in pharmaceutical formulations. APIs can come from a range of processes, such as fermentation, natural resource isolation, or synthetic processes. Their potency, stability, and purity are essential for guaranteeing the final drug product's safety and effectiveness.
According to the World Health Organization (WHO), access to essential medicines, including Active Pharmaceutical Ingredients (APIs), is a fundamental human right, essential for achieving the highest attainable standard of health.

Market Dynamics:
Driver:
Growing need for prescription drugs
Generic pharmaceutical companies are seeing an opening to enter the market with less expensive alternatives as a result of branded drug patents expiring. The substantial cost savings that generic medications provide to patients and healthcare systems encourage their widespread use. Generic versions of previously patented drugs are being allowed to enter the market thanks to regulatory pathways for approval, such as the US Abbreviated New Drug Applications (ANDAs). Furthermore, when affordable generic substitutes are available, the use of them is encouraged by generic drug substitution policies put in place by healthcare payers and providers.
Restraint:
Price decline and cost constraints
API manufacturers frequently face margin pressure and price erosion as a result of the fierce competition in the pharmaceutical industry, especially in the generic drug segment. Prices are trending lower as a result of price competition between buyers and sellers, industry consolidation, and government programs to control healthcare costs. Additionally, in order to stay profitable in the face of price pressure, API manufacturers need to constantly improve their operations, increase efficiency, and look into ways to cut costs.
Opportunity:
Growth of customized health care
API producers can greatly benefit from personalized medicine strategies, which center on adjusting medical care to the unique needs of each patient. The development of more effective and less side-effect-prone targeted therapies is made possible by developments in genomic sequencing, biomarker identification, and data analytics. Furthermore, personalized medicine applications may employ targeted therapeutics, gene editing technologies, and companion diagnostics as APIs. To create and produce customized medical products, API manufacturers can work with pharmaceutical, diagnostic, and healthcare organizations.
Threat:
Strong rivalry and pricing pressure
There is fierce competition in the API manufacturing sector, both nationally and internationally. As a result of competition among manufacturers for market share, margins are compressed and prices are eroded. In the generic API market, where businesses mainly compete on price, this pressure is especially intense. Moreover, the existence of low-cost manufacturers in areas with cheaper labor and production costs heightens competition, making it difficult for businesses to continue growing and remaining profitable.
Covid-19 Impact:
The COVID-19 pandemic has caused significant disruptions in the pharmaceutical supply chain and has had a significant effect on the API market. Production delays and supply chain bottlenecks have resulted from disruptions in manufacturing operations caused by lockdown measures, travel restrictions, and workforce shortages. The demand for vital drugs, like steroids, antibiotics, and antivirals, has increased. This has put pressure on supply chains and made shortages of finished dosage forms and vital APIs worse. Additionally, pharmaceutical companies have encountered difficulties in locating raw materials, shipping products, and preserving business continuity in the face of uncertainties brought on by pandemics.
The Branded or Innovative Prescription Drugs segment is expected to be the largest during the forecast period
In the Active Pharmaceutical Ingredients (API) market, the branded or innovative prescription drug segment is projected to hold the largest share. Novel compounds and intricate synthesis procedures are in high demand since branded prescription medications frequently need unique and proprietary APIs. One reason for these medications' higher market value and share is that they usually go through lengthy clinical trials, regulatory approval procedures, and research and development before going on sale. Furthermore, to fill gaps in the medical field and set themselves apart from competitors, pharmaceutical companies heavily invest in the development of novel therapies.
The Oncology segment is expected to have the highest CAGR during the forecast period
It is projected that the market for active pharmaceutical ingredients (APIs) will have the highest CAGR in the oncology segment. Due to the rising global incidence of cancer and the ongoing development of novel cancer therapies, oncology APIs are in high demand. The APIs utilized in the treatment of solid tumors and hematologic malignancies fall under the category of oncology. Moreover, novel cancer treatments that rely on specific APIs have been developed as a result of advancements in molecular biology, targeted therapies, and immunotherapy.
Region with largest share:
It is projected that the market for active pharmaceutical ingredients (APIs) will hold the largest share in the North American region. Many factors contribute to this region's dominance, such as a strong pharmaceutical industry, large investments in R&D, sophisticated manufacturing infrastructure, and strict regulatory requirements. Additionally, North America is the market leader due in part to the existence of top pharmaceutical companies, contract manufacturing organizations (CMOs), and API manufacturers. Demand for APIs used in prescription medications is further driven by the region's large patient population, high healthcare expenditures, and advantageous reimbursement policies.
Region with highest CAGR:
It is anticipated that the market for active pharmaceutical ingredients (APIs) will grow at the highest CAGR in Europe. A robust regulatory framework, sophisticated manufacturing capabilities, and a well-established pharmaceutical industry are some of the factors propelling the growth of the API market in Europe. The area gains from a strong ecosystem for research and development, which promotes creativity and the creation of cutting-edge medication treatments that depend on specialized APIs. Furthermore, the emphasis Europe has placed on affordable and accessible healthcare, along with the growing need for generic drugs, all support the growth of the API market.
Key players in the market
Some of the key players in Active Pharmaceutical Ingredients market include BASF SE, Dr. Reddy's Laboratories Ltd, Cambrex Corporation, Abbott, Cipla Inc., GlaxoSmithKline plc, Amgen Inc., Merck & Co., Inc., AbbVie, Inc., Mylan N.V., Bausch Health Companies Inc., Eli Lilly and Company, Biocon Ltd., Johnson & Johnson Private Limited, AstraZeneca, Takeda Pharmaceutical Company Limited, Novartis AG, Teva Pharmaceutical Industries Ltd., Sun Pharmaceutical Industries Ltd and Pfizer, Inc.
Key Developments:
In April 2024, BASF signed a 25-year power purchase agreement (PPA) with China Energy Engineering Group Guangdong Electric Power Design Institute Co., Ltd. (GEDI) to purchase renewable electricity for its Zhanjiang Verbund site. The PPA is a further step in the renewable energy partnership between BASF and GEDI following the Letter of Intent (LOI).
In March 2024, Dr. Reddy's Laboratories said that it has entered into a license agreement with Pharmazz Inc. to commercialise the first-in-class innovative drug Centhaquine in India. Pharmazz is a U.S. based biopharmaceutical company developing and commercializing drug products to treat critically ill patients.
In September 2023, Abbott has entered a definitive agreement for the acquisition of Bigfoot Biomedical, which develops smart insulin management systems for individuals with diabetes. Together, the companies have worked on connected diabetes solutions since 2017.
Drug Types Covered:
• Branded or Innovative Prescription Drugs
• Generic Prescription Drugs
• Over-the-counter (OTC) Drugs
• Other Drug Types
Manufacturer Types Covered:
• In House Manufacturing
• Contract Manufacturing
• Other Manufacturer Types
API Types Covered:
• Synthetic
• Biological
• Plant Extracts
• Other API Types
Applications Covered:
• Anti-infective
• Cardiovascular
• Metabolic Disorder
• Respiratory
• Ophthalmology
• Neurology
• Oncology
• Other Applications
End Users Covered:
• Pharmaceutical & Biotechnological Industry
• CROs
• CMOs
• Other End Users
Regions Covered:
• North America
o US
o Canada
o Mexico
• Europe
o Germany
o UK
o Italy
o France
o Spain
o Rest of Europe
• Asia Pacific
o Japan
o China
o India
o Australia
o New Zealand
o South Korea
o Rest of Asia Pacific
• South America
o Argentina
o Brazil
o Chile
o Rest of South America
• Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Rest of Middle East & Africa
What our report offers:
- Market share assessments for the regional and country-level segments
- Strategic recommendations for the new entrants
- Covers Market data for the years 2021, 2022, 2023, 2026, and 2030
- Market Trends (Drivers, Constraints, Opportunities, Threats, Challenges, Investment Opportunities, and recommendations)
- Strategic recommendations in key business segments based on the market estimations
- Competitive landscaping mapping the key common trends
- Company profiling with detailed strategies, financials, and recent developments
- Supply chain trends mapping the latest technological advancements
Free Customization Offerings:
All the customers of this report will be entitled to receive one of the following free customization options:
• Company Profiling
o Comprehensive profiling of additional market players (up to 3)
o SWOT Analysis of key players (up to 3)
• Regional Segmentation
o Market estimations, Forecasts and CAGR of any prominent country as per the client's interest (Note: Depends on feasibility check)
• Competitive Benchmarking
Benchmarking of key players based on product portfolio, geographical presence, and strategic alliances
Table of Contents
1 Executive Summary
2 Preface
2.1 Abstract
2.2 Stake Holders
2.3 Research Scope
2.4 Research Methodology
2.4.1 Data Mining
2.4.2 Data Analysis
2.4.3 Data Validation
2.4.4 Research Approach
2.5 Research Sources
2.5.1 Primary Research Sources
2.5.2 Secondary Research Sources
2.5.3 Assumptions
3 Market Trend Analysis
3.1 Introduction
3.2 Drivers
3.3 Restraints
3.4 Opportunities
3.5 Threats
3.6 Application Analysis
3.7 End User Analysis
3.8 Emerging Markets
3.9 Impact of Covid-19
4 Porters Five Force Analysis
4.1 Bargaining power of suppliers
4.2 Bargaining power of buyers
4.3 Threat of substitutes
4.4 Threat of new entrants
4.5 Competitive rivalry
5 Global Active Pharmaceutical Ingredients Market, By Drug Type
5.1 Introduction
5.2 Branded or Innovative Prescription Drugs
5.3 Generic Prescription Drugs
5.4 Over-the-counter (OTC) Drugs
5.5 Other Drug Types
6 Global Active Pharmaceutical Ingredients Market, By Manufacturer Type
6.1 Introduction
6.2 In House Manufacturing
6.3 Contract Manufacturing
6.4 Other Manufacturer Types
7 Global Active Pharmaceutical Ingredients Market, By API Type
7.1 Introduction
7.2 Synthetic
7.3 Biological
7.4 Plant Extracts
7.5 Other API Types
8 Global Active Pharmaceutical Ingredients Market, By Application
8.1 Introduction
8.2 Anti-infective
8.3 Cardiovascular
8.4 Metabolic Disorder
8.5 Respiratory
8.6 Ophthalmology
8.7 Neurology
8.8 Oncology
8.9 Other Applications
9 Global Active Pharmaceutical Ingredients Market, By End User
9.1 Introduction
9.2 Pharmaceutical & Biotechnological Industry
9.3 CROs
9.4 CMOs
9.5 Other End Users
10 Global Active Pharmaceutical Ingredients Market, By Geography
10.1 Introduction
10.2 North America
10.2.1 US
10.2.2 Canada
10.2.3 Mexico
10.3 Europe
10.3.1 Germany
10.3.2 UK
10.3.3 Italy
10.3.4 France
10.3.5 Spain
10.3.6 Rest of Europe
10.4 Asia Pacific
10.4.1 Japan
10.4.2 China
10.4.3 India
10.4.4 Australia
10.4.5 New Zealand
10.4.6 South Korea
10.4.7 Rest of Asia Pacific
10.5 South America
10.5.1 Argentina
10.5.2 Brazil
10.5.3 Chile
10.5.4 Rest of South America
10.6 Middle East & Africa
10.6.1 Saudi Arabia
10.6.2 UAE
10.6.3 Qatar
10.6.4 South Africa
10.6.5 Rest of Middle East & Africa
11 Key Developments
11.1 Agreements, Partnerships, Collaborations and Joint Ventures
11.2 Acquisitions & Mergers
11.3 New Product Launch
11.4 Expansions
11.5 Other Key Strategies
12 Company Profiling
12.1 BASF SE
12.2 Dr. Reddy's Laboratories Ltd
12.3 Cambrex Corporation
12.4 Abbott
12.5 Cipla Inc.
12.6 GlaxoSmithKline plc
12.7 Amgen Inc.
12.8 Merck & Co., Inc.
12.9 AbbVie, Inc.
12.10 Mylan N.V.
12.11 Bausch Health Companies Inc.
12.12 Eli Lilly and Company
12.13 Biocon Ltd.
12.14 Johnson & Johnson Private Limited
12.15 AstraZeneca
12.16 Takeda Pharmaceutical Company Limited
12.17 Novartis AG
12.18 Teva Pharmaceutical Industries Ltd.
12.19 Sun Pharmaceutical Industries Ltd
12.20 Pfizer, Inc.
List of Tables
1 Global Active Pharmaceutical Ingredients Market Outlook, By Region (2021-2030) ($MN)
2 Global Active Pharmaceutical Ingredients Market Outlook, By Drug Type (2021-2030) ($MN)
3 Global Active Pharmaceutical Ingredients Market Outlook, By Branded or Innovative Prescription Drugs (2021-2030) ($MN)
4 Global Active Pharmaceutical Ingredients Market Outlook, By Generic Prescription Drugs (2021-2030) ($MN)
5 Global Active Pharmaceutical Ingredients Market Outlook, By Over-the-counter (OTC) Drugs (2021-2030) ($MN)
6 Global Active Pharmaceutical Ingredients Market Outlook, By Other Drug Types (2021-2030) ($MN)
7 Global Active Pharmaceutical Ingredients Market Outlook, By Manufacturer Type (2021-2030) ($MN)
8 Global Active Pharmaceutical Ingredients Market Outlook, By In House Manufacturing (2021-2030) ($MN)
9 Global Active Pharmaceutical Ingredients Market Outlook, By Contract Manufacturing (2021-2030) ($MN)
10 Global Active Pharmaceutical Ingredients Market Outlook, By Other Manufacturer Types (2021-2030) ($MN)
11 Global Active Pharmaceutical Ingredients Market Outlook, By API Type (2021-2030) ($MN)
12 Global Active Pharmaceutical Ingredients Market Outlook, By Synthetic (2021-2030) ($MN)
13 Global Active Pharmaceutical Ingredients Market Outlook, By Biological (2021-2030) ($MN)
14 Global Active Pharmaceutical Ingredients Market Outlook, By Plant Extracts (2021-2030) ($MN)
15 Global Active Pharmaceutical Ingredients Market Outlook, By Other API Types (2021-2030) ($MN)
16 Global Active Pharmaceutical Ingredients Market Outlook, By Application (2021-2030) ($MN)
17 Global Active Pharmaceutical Ingredients Market Outlook, By Anti-infective (2021-2030) ($MN)
18 Global Active Pharmaceutical Ingredients Market Outlook, By Cardiovascular (2021-2030) ($MN)
19 Global Active Pharmaceutical Ingredients Market Outlook, By Metabolic Disorder (2021-2030) ($MN)
20 Global Active Pharmaceutical Ingredients Market Outlook, By Respiratory (2021-2030) ($MN)
21 Global Active Pharmaceutical Ingredients Market Outlook, By Ophthalmology (2021-2030) ($MN)
22 Global Active Pharmaceutical Ingredients Market Outlook, By Neurology (2021-2030) ($MN)
23 Global Active Pharmaceutical Ingredients Market Outlook, By Oncology (2021-2030) ($MN)
24 Global Active Pharmaceutical Ingredients Market Outlook, By Other Applications (2021-2030) ($MN)
25 Global Active Pharmaceutical Ingredients Market Outlook, By End User (2021-2030) ($MN)
26 Global Active Pharmaceutical Ingredients Market Outlook, By Pharmaceutical & Biotechnological Industry (2021-2030) ($MN)
27 Global Active Pharmaceutical Ingredients Market Outlook, By CROs (2021-2030) ($MN)
28 Global Active Pharmaceutical Ingredients Market Outlook, By CMOs (2021-2030) ($MN)
29 Global Active Pharmaceutical Ingredients Market Outlook, By Other End Users (2021-2030) ($MN)
Note: Tables for North America, Europe, APAC, South America, and Middle East & Africa Regions are also represented in the same manner as above.
List of Figures
RESEARCH METHODOLOGY
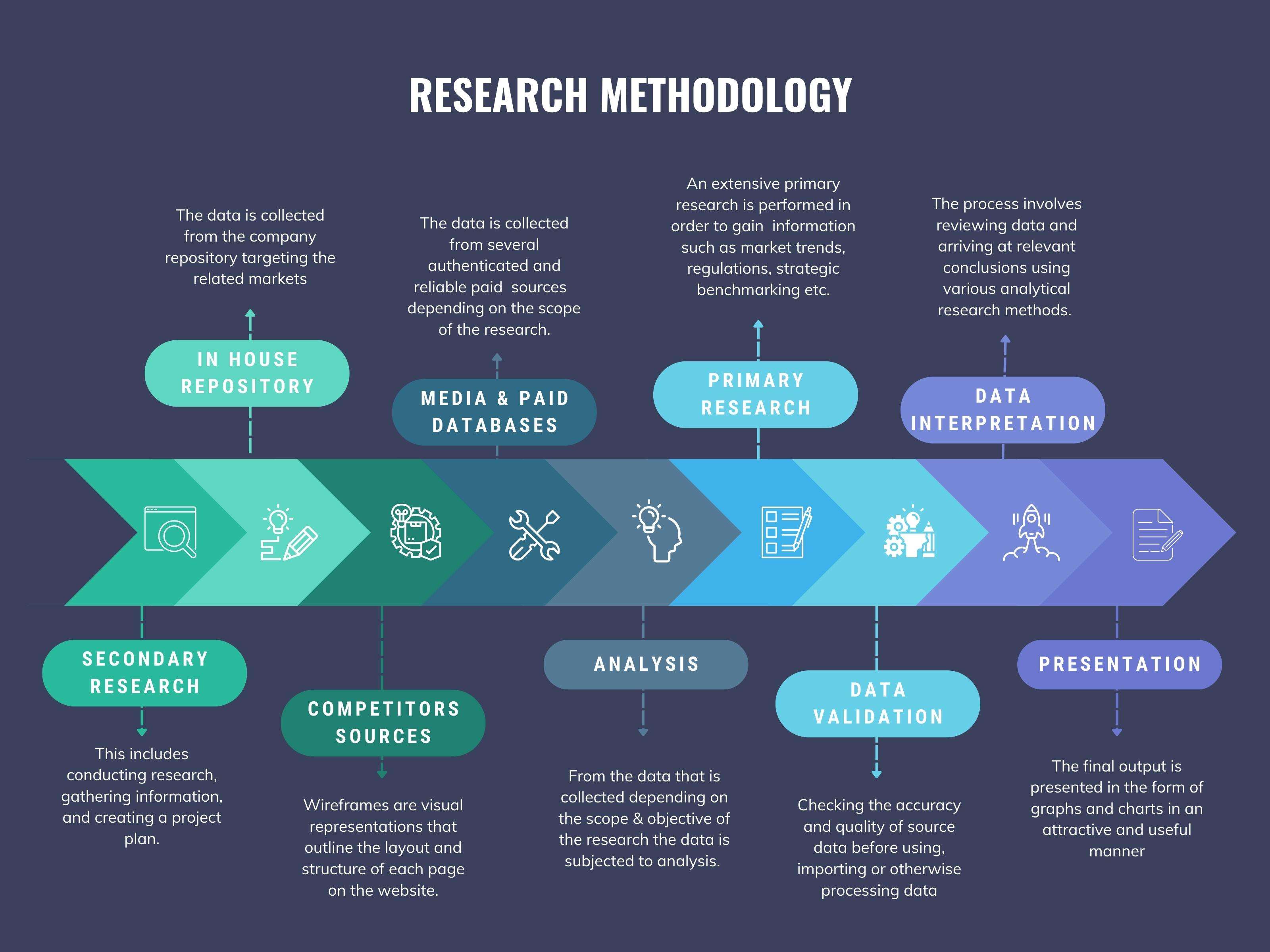
We at ‘Stratistics’ opt for an extensive research approach which involves data mining, data validation, and data analysis. The various research sources include in-house repository, secondary research, competitor’s sources, social media research, client internal data, and primary research.
Our team of analysts prefers the most reliable and authenticated data sources in order to perform the comprehensive literature search. With access to most of the authenticated data bases our team highly considers the best mix of information through various sources to obtain extensive and accurate analysis.
Each report takes an average time of a month and a team of 4 industry analysts. The time may vary depending on the scope and data availability of the desired market report. The various parameters used in the market assessment are standardized in order to enhance the data accuracy.
Data Mining
The data is collected from several authenticated, reliable, paid and unpaid sources and is filtered depending on the scope & objective of the research. Our reports repository acts as an added advantage in this procedure. Data gathering from the raw material suppliers, distributors and the manufacturers is performed on a regular basis, this helps in the comprehensive understanding of the products value chain. Apart from the above mentioned sources the data is also collected from the industry consultants to ensure the objective of the study is in the right direction.
Market trends such as technological advancements, regulatory affairs, market dynamics (Drivers, Restraints, Opportunities and Challenges) are obtained from scientific journals, market related national & international associations and organizations.
Data Analysis
From the data that is collected depending on the scope & objective of the research the data is subjected for the analysis. The critical steps that we follow for the data analysis include:
- Product Lifecycle Analysis
- Competitor analysis
- Risk analysis
- Porters Analysis
- PESTEL Analysis
- SWOT Analysis
The data engineering is performed by the core industry experts considering both the Marketing Mix Modeling and the Demand Forecasting. The marketing mix modeling makes use of multiple-regression techniques to predict the optimal mix of marketing variables. Regression factor is based on a number of variables and how they relate to an outcome such as sales or profits.
Data Validation
The data validation is performed by the exhaustive primary research from the expert interviews. This includes telephonic interviews, focus groups, face to face interviews, and questionnaires to validate our research from all aspects. The industry experts we approach come from the leading firms, involved in the supply chain ranging from the suppliers, distributors to the manufacturers and consumers so as to ensure an unbiased analysis.
We are in touch with more than 15,000 industry experts with the right mix of consultants, CEO's, presidents, vice presidents, managers, experts from both supply side and demand side, executives and so on.
The data validation involves the primary research from the industry experts belonging to:
- Leading Companies
- Suppliers & Distributors
- Manufacturers
- Consumers
- Industry/Strategic Consultants
Apart from the data validation the primary research also helps in performing the fill gap research, i.e. providing solutions for the unmet needs of the research which helps in enhancing the reports quality.
For more details about research methodology, kindly write to us at info@strategymrc.com
Frequently Asked Questions
In case of any queries regarding this report, you can contact the customer service by filing the “Inquiry Before Buy” form available on the right hand side. You may also contact us through email: info@strategymrc.com or phone: +1-301-202-5929
Yes, the samples are available for all the published reports. You can request them by filling the “Request Sample” option available in this page.
Yes, you can request a sample with your specific requirements. All the customized samples will be provided as per the requirement with the real data masked.
All our reports are available in Digital PDF format. In case if you require them in any other formats, such as PPT, Excel etc you can submit a request through “Inquiry Before Buy” form available on the right hand side. You may also contact us through email: info@strategymrc.com or phone: +1-301-202-5929
We offer a free 15% customization with every purchase. This requirement can be fulfilled for both pre and post sale. You may send your customization requirements through email at info@strategymrc.com or call us on +1-301-202-5929.
We have 3 different licensing options available in electronic format.
- Single User Licence: Allows one person, typically the buyer, to have access to the ordered product. The ordered product cannot be distributed to anyone else.
- 2-5 User Licence: Allows the ordered product to be shared among a maximum of 5 people within your organisation.
- Corporate License: Allows the product to be shared among all employees of your organisation regardless of their geographical location.
All our reports are typically be emailed to you as an attachment.
To order any available report you need to register on our website. The payment can be made either through CCAvenue or PayPal payments gateways which accept all international cards.
We extend our support to 6 months post sale. A post sale customization is also provided to cover your unmet needs in the report.
Request Customization
We offer complimentary customization of up to 15% with every purchase. To share your customization requirements, feel free to email us at info@strategymrc.com or call us on +1-301-202-5929. .
Please Note: Customization within the 15% threshold is entirely free of charge. If your request exceeds this limit, we will conduct a feasibility assessment. Following that, a detailed quote and timeline will be provided.
WHY CHOOSE US ?

Assured Quality
Best in class reports with high standard of research integrity

24X7 Research Support
Continuous support to ensure the best customer experience.

Free Customization
Adding more values to your product of interest.

Safe & Secure Access
Providing a secured environment for all online transactions.

Trusted by 600+ Brands
Serving the most reputed brands across the world.
