
Biologics And Biosimilars Market
Biologics & Biosimilars Market Forecasts to 2032 – Global Analysis By Product Type (Biologics, Biosimilars), Therapeutic Area (Oncology, Ophthalmology, Autoimmune Disorders, Cardiovascular, Endocrinology, Infectious Diseases, Hematology, and Wound Healing & Regenerative Care), Manufacturing Source, Distribution Channel, End User, and By Geography

According to Stratistics MRC, the Global Biologics & Biosimilars Market is accounted for $538.20 billion in 2025 and is expected to reach $1219.86 billion by 2032 growing at a CAGR of 12.4% during the forecast period. Biologics are innovative therapies produced using living cells or organisms, such as monoclonal antibodies, proteins, and vaccines, that target chronic and severe diseases. Biosimilars, created after biologics lose patent protection, are close replicas in terms of safety, efficacy, and quality, ensuring similar therapeutic effects. These cost-effective options expand patient access to critical treatments. Together, biologics and biosimilars enhance healthcare by advancing medical solutions, reducing treatment costs, and improving overall clinical outcomes.
Market Dynamics:
Driver:
Rising prevalence of chronic diseases
The increasing global burden of chronic illnesses such as cancer, diabetes, and autoimmune disorders is fuelling demand for biologics and biosimilars. Healthcare systems are prioritizing long-term treatment solutions that offer improved efficacy and reduced side effects. Biologics, with their targeted mechanisms, are becoming central to disease management strategies. As patient population’s age and diagnostic capabilities improve, the need for biologic therapies continues to rise. Biosimilars are gaining traction as cost-effective alternatives, especially in markets with strained healthcare budgets. This growing demand is accelerating innovation and investment across the biopharmaceutical landscape.
Restraint:
High development and manufacturing costs
Developing biologics and biosimilars involves complex processes, from cell line engineering to purification and validation. These high costs are compounded by stringent regulatory requirements and extended clinical trial timelines. Manufacturing facilities must meet rigorous quality standards, often requiring specialized infrastructure and skilled personnel. The need for cold chain logistics and contamination control further increases operational expenses. Smaller firms face barriers to entry, limiting competition and slowing market expansion. As a result, pricing remains a challenge, especially in emerging economies.
Opportunity:
Technological advancements in R&D and manufacturing
Advanced analytics and AI-driven modelling are enhancing drug discovery and reducing development timelines. Innovations in cell culture media and expression systems are improving yield and product consistency. Automation and digital twins are being adopted to optimize facility operations and reduce human error. These advancements are lowering costs and enabling scalable production of complex biologics. As technology matures, more players can enter the market with competitive biosimilar offerings.
Threat:
Intense competition and pricing pressure
Patent expirations are opening the door to multiple entrants, intensifying pricing battles. Regulatory pathways, while evolving, still pose hurdles that delay market access and increase compliance costs. Payers and healthcare providers are demanding lower prices, squeezing margins across the value chain. Brand loyalty and physician hesitancy toward biosimilars also impact adoption rates. Without differentiation and strategic positioning, companies risk losing market share in an increasingly commoditized landscape.
Covid-19 Impact
The pandemic disrupted clinical trials, supply chains, and manufacturing schedules for biologics and biosimilars. Lockdowns and resource reallocation toward vaccines and emergency treatments delayed non-COVID product launches. However, the crisis also highlighted the importance of biologics in managing infectious diseases and immune responses. Biopharma companies accelerated digital transformation, adopting remote monitoring and decentralized trial models. Governments and regulators introduced flexible frameworks to maintain continuity in drug development. Post-pandemic strategies now emphasize resilience, agility, and global collaboration in biologics manufacturing.
The biologics segment is expected to be the largest during the forecast period
The biologics segment is expected to account for the largest market share during the forecast period, its superior therapeutic outcomes and expanding indications. Monoclonal antibodies, recombinant proteins, and gene therapies are gaining widespread clinical acceptance. Increasing prevalence of complex diseases is driving demand for targeted biologic treatments. Regulatory approvals are accelerating, supported by robust clinical data and patient advocacy. Investment in biologics R&D continues to outpace other segments, reinforcing its market leadership. As biologics become more accessible, their share in global pharmaceutical spending is expected to rise steadily.
The biopharma companies segment is expected to have the highest CAGR during the forecast period
Over the forecast period, the biopharma companies segment is predicted to witness the highest growth rate, driven by aggressive innovation and pipeline expansion. These firms are leveraging advanced platforms for biologic synthesis, formulation, and delivery. Strategic collaborations and licensing agreements are enabling faster market entry and broader geographic reach. Biopharma players are also investing in biosimilar portfolios to capture post-patent opportunities. Their agility and focus on niche therapies position them well for sustained growth. As demand for personalized medicine increases, biopharma companies are leading the charge in biologics development.
Region with largest share:
During the forecast period, the Asia Pacific region is expected to hold the largest market share supported by rising healthcare expenditure and expanding patient populations. Countries like China, India, and South Korea are investing heavily in biologics infrastructure and regulatory modernization. Local manufacturers are scaling up production to meet domestic and export demand. Government initiatives are promoting biosimilar adoption to reduce treatment costs. Clinical trial activity is surging, with Asia Pacific emerging as a hub for biologics research. The region’s demographic and economic dynamics make it a key growth engine for the industry.
Region with highest CAGR:
Over the forecast period, the North America region is anticipated to exhibit the highest CAGR, furled by technological leadership and robust biopharma investment. The U.S. and Canada are advancing biologics innovation through academic-industry partnerships and federal funding. Regulatory agencies are streamlining approval pathways for biosimilars, encouraging market competition. Adoption of precision medicine and biologic therapies is accelerating across therapeutic areas. Digital health integration and AI-driven drug development are enhancing efficiency and outcomes.
Key players in the market
Some of the key players profiled in the Biologics & Biosimilars Market include Amgen, Sanofi, Pfizer, Eli Lilly, Novartis, Viatris, Biocon Biologics, AbbVie, Samsung Bioepis, Teva Pharmaceuticals, Celltrion, Fresenius Kabi, Roche, Boehringer Ingelheim, and Merck KGaA.
Key Developments:
In September 2025, Novartis AG has reached an agreement to purchase the New York-based firm, Tourmaline Bio Inc., in a deal valued at a staggering $1.4 billion. This move is aimed at reducing systemic inflammation, which is termed a major driver of cardiovascular disease. Novartis has been on the lookout for deals that would amplify its sales beyond 2025.
In August 2025, Sanofi announces the completion of its acquisition of Vigil Neuroscience, Inc. This acquisition strengthens Sanofi’s early-stage pipeline in neurology with VG-3927, a novel, oral, small-molecule TREM2 agonist, which will be evaluated in a phase 2 clinical study in patients with Alzheimer’s disease. In addition, the acquisition of Vigil’s preclinical pipeline will further strengthen Sanofi’s research in various neurodegenerative diseases.
Product Types Covered:
• Biologics
• Biosimilars
Therapeutic Areas Covered:
• Oncology
• Ophthalmology
• Autoimmune Disorders
• Cardiovascular
• Endocrinology
• Infectious Diseases
• Hematology
• Wound Healing & Regenerative Care
Manufacturing Sources Covered:
• Mammalian Cell Culture
• Yeast Expression Systems
• Bacterial Cell Culture
• Transgenic Models
Distribution Channels Covered:
• Hospital Pharmacies
• Specialty Clinics
• Retail Pharmacies
• Online Pharmacies
End Users Covered:
• Hospitals
• Diagnostic Labs
• Research Institutes
• Biopharma Companies
• Other End Users
Regions Covered:
• North America
o US
o Canada
o Mexico
• Europe
o Germany
o UK
o Italy
o France
o Spain
o Rest of Europe
• Asia Pacific
o Japan
o China
o India
o Australia
o New Zealand
o South Korea
o Rest of Asia Pacific
• South America
o Argentina
o Brazil
o Chile
o Rest of South America
• Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Rest of Middle East & Africa
What our report offers:
- Market share assessments for the regional and country-level segments
- Strategic recommendations for the new entrants
- Covers Market data for the years 2024, 2025, 2026, 2028, and 2032
- Market Trends (Drivers, Constraints, Opportunities, Threats, Challenges, Investment Opportunities, and recommendations)
- Strategic recommendations in key business segments based on the market estimations
- Competitive landscaping mapping the key common trends
- Company profiling with detailed strategies, financials, and recent developments
- Supply chain trends mapping the latest technological advancements
Free Customization Offerings:
All the customers of this report will be entitled to receive one of the following free customization options:
• Company Profiling
o Comprehensive profiling of additional market players (up to 3)
o SWOT Analysis of key players (up to 3)
• Regional Segmentation
o Market estimations, Forecasts and CAGR of any prominent country as per the client's interest (Note: Depends on feasibility check)
• Competitive Benchmarking
o Benchmarking of key players based on product portfolio, geographical presence, and strategic alliances
Table of Contents
1 Executive Summary
2 Preface
2.1 Abstract
2.2 Stake Holders
2.3 Research Scope
2.4 Research Methodology
2.4.1 Data Mining
2.4.2 Data Analysis
2.4.3 Data Validation
2.4.4 Research Approach
2.5 Research Sources
2.5.1 Primary Research Sources
2.5.2 Secondary Research Sources
2.5.3 Assumptions
3 Market Trend Analysis
3.1 Introduction
3.2 Drivers
3.3 Restraints
3.4 Opportunities
3.5 Threats
3.6 Product Analysis
3.7 End User Analysis
3.8 Emerging Markets
3.9 Impact of Covid-19
4 Porters Five Force Analysis
4.1 Bargaining power of suppliers
4.2 Bargaining power of buyers
4.3 Threat of substitutes
4.4 Threat of new entrants
4.5 Competitive rivalry
5 Global Biologics & Biosimilars Market, By Product Type
5.1 Introduction
5.2 Biologics
5.2.1 Monoclonal Antibodies
5.2.2 Recombinant Proteins
5.2.3 Hormones
5.2.4 Vaccines
5.2.5 Cytokines & Interferons
5.2.6 Fusion Proteins
5.3 Biosimilars
5.3.1 Monoclonal Antibody Biosimilars
5.3.2 Low Molecular Weight Heparins
5.3.3 Recombinant Hormone Biosimilars
5.3.4 Immunomodulators
5.3.5 Anti-inflammatory Biosimilars
6 Global Biologics & Biosimilars Market, By Therapeutic Area
6.1 Introduction
6.2 Oncology
6.3 Ophthalmology
6.4 Autoimmune Disorders
6.5 Cardiovascular
6.6 Endocrinology
6.7 Infectious Diseases
6.8 Hematology
6.9 Wound Healing & Regenerative Care
7 Global Biologics & Biosimilars Market, By Manufacturing Source
7.1 Introduction
7.2 Mammalian Cell Culture
7.3 Yeast Expression Systems
7.4 Bacterial Cell Culture
7.5 Transgenic Models
8 Global Biologics & Biosimilars Market, By Distribution Channel
8.1 Introduction
8.2 Hospital Pharmacies
8.3 Specialty Clinics
8.4 Retail Pharmacies
8.5 Online Pharmacies
9 Global Biologics & Biosimilars Market, By End User
9.1 Introduction
9.2 Hospitals
9.3 Diagnostic Labs
9.4 Research Institutes
9.5 Biopharma Companies
9.6 Other End Users
10 Global Biologics & Biosimilars Market, By Geography
10.1 Introduction
10.2 North America
10.2.1 US
10.2.2 Canada
10.2.3 Mexico
10.3 Europe
10.3.1 Germany
10.3.2 UK
10.3.3 Italy
10.3.4 France
10.3.5 Spain
10.3.6 Rest of Europe
10.4 Asia Pacific
10.4.1 Japan
10.4.2 China
10.4.3 India
10.4.4 Australia
10.4.5 New Zealand
10.4.6 South Korea
10.4.7 Rest of Asia Pacific
10.5 South America
10.5.1 Argentina
10.5.2 Brazil
10.5.3 Chile
10.5.4 Rest of South America
10.6 Middle East & Africa
10.6.1 Saudi Arabia
10.6.2 UAE
10.6.3 Qatar
10.6.4 South Africa
10.6.5 Rest of Middle East & Africa
11 Key Developments
11.1 Agreements, Partnerships, Collaborations and Joint Ventures
11.2 Acquisitions & Mergers
11.3 New Product Launch
11.4 Expansions
11.5 Other Key Strategies
12 Company Profiling
12.1 Amgen
12.2 Sanofi
12.3 Pfizer
12.4 Eli Lilly
12.5 Novartis
12.6 Viatris
12.7 Biocon Biologics
12.8 AbbVie
12.9 Samsung Bioepis
12.10 Teva Pharmaceuticals
12.11 Celltrion
12.12 Fresenius Kabi
12.13 Roche
12.14 Boehringer Ingelheim
12.15 Merck KGaA
List of Tables
1 Global Biologics & Biosimilars Market Outlook, By Region (2024-2032) ($MN)
2 Global Biologics & Biosimilars Market Outlook, By Product Type (2024-2032) ($MN)
3 Global Biologics & Biosimilars Market Outlook, By Biologics (2024-2032) ($MN)
4 Global Biologics & Biosimilars Market Outlook, By Monoclonal Antibodies (2024-2032) ($MN)
5 Global Biologics & Biosimilars Market Outlook, By Recombinant Proteins (2024-2032) ($MN)
6 Global Biologics & Biosimilars Market Outlook, By Hormones (2024-2032) ($MN)
7 Global Biologics & Biosimilars Market Outlook, By Vaccines (2024-2032) ($MN)
8 Global Biologics & Biosimilars Market Outlook, By Cytokines & Interferons (2024-2032) ($MN)
9 Global Biologics & Biosimilars Market Outlook, By Fusion Proteins (2024-2032) ($MN)
10 Global Biologics & Biosimilars Market Outlook, By Biosimilars (2024-2032) ($MN)
11 Global Biologics & Biosimilars Market Outlook, By Monoclonal Antibody Biosimilars (2024-2032) ($MN)
12 Global Biologics & Biosimilars Market Outlook, By Low Molecular Weight Heparins (2024-2032) ($MN)
13 Global Biologics & Biosimilars Market Outlook, By Recombinant Hormone Biosimilars (2024-2032) ($MN)
14 Global Biologics & Biosimilars Market Outlook, By Immunomodulators (2024-2032) ($MN)
15 Global Biologics & Biosimilars Market Outlook, By Anti-inflammatory Biosimilars (2024-2032) ($MN)
16 Global Biologics & Biosimilars Market Outlook, By Therapeutic Area (2024-2032) ($MN)
17 Global Biologics & Biosimilars Market Outlook, By Oncology (2024-2032) ($MN)
18 Global Biologics & Biosimilars Market Outlook, By Ophthalmology (2024-2032) ($MN)
19 Global Biologics & Biosimilars Market Outlook, By Autoimmune Disorders (2024-2032) ($MN)
20 Global Biologics & Biosimilars Market Outlook, By Cardiovascular (2024-2032) ($MN)
21 Global Biologics & Biosimilars Market Outlook, By Endocrinology (2024-2032) ($MN)
22 Global Biologics & Biosimilars Market Outlook, By Infectious Diseases (2024-2032) ($MN)
23 Global Biologics & Biosimilars Market Outlook, By Hematology (2024-2032) ($MN)
24 Global Biologics & Biosimilars Market Outlook, By Wound Healing & Regenerative Care (2024-2032) ($MN)
25 Global Biologics & Biosimilars Market Outlook, By Manufacturing Source (2024-2032) ($MN)
26 Global Biologics & Biosimilars Market Outlook, By Mammalian Cell Culture (2024-2032) ($MN)
27 Global Biologics & Biosimilars Market Outlook, By Yeast Expression Systems (2024-2032) ($MN)
28 Global Biologics & Biosimilars Market Outlook, By Bacterial Cell Culture (2024-2032) ($MN)
29 Global Biologics & Biosimilars Market Outlook, By Transgenic Models (2024-2032) ($MN)
30 Global Biologics & Biosimilars Market Outlook, By Distribution Channel (2024-2032) ($MN)
31 Global Biologics & Biosimilars Market Outlook, By Hospital Pharmacies (2024-2032) ($MN)
32 Global Biologics & Biosimilars Market Outlook, By Specialty Clinics (2024-2032) ($MN)
33 Global Biologics & Biosimilars Market Outlook, By Retail Pharmacies (2024-2032) ($MN)
34 Global Biologics & Biosimilars Market Outlook, By Online Pharmacies (2024-2032) ($MN)
35 Global Biologics & Biosimilars Market Outlook, By End User (2024-2032) ($MN)
36 Global Biologics & Biosimilars Market Outlook, By Hospitals (2024-2032) ($MN)
37 Global Biologics & Biosimilars Market Outlook, By Diagnostic Labs (2024-2032) ($MN)
38 Global Biologics & Biosimilars Market Outlook, By Research Institutes (2024-2032) ($MN)
39 Global Biologics & Biosimilars Market Outlook, By Biopharma Companies (2024-2032) ($MN)
40 Global Biologics & Biosimilars Market Outlook, By Other End Users (2024-2032) ($MN)
Note: Tables for North America, Europe, APAC, South America, and Middle East & Africa Regions are also represented in the same manner as above.
List of Figures
RESEARCH METHODOLOGY
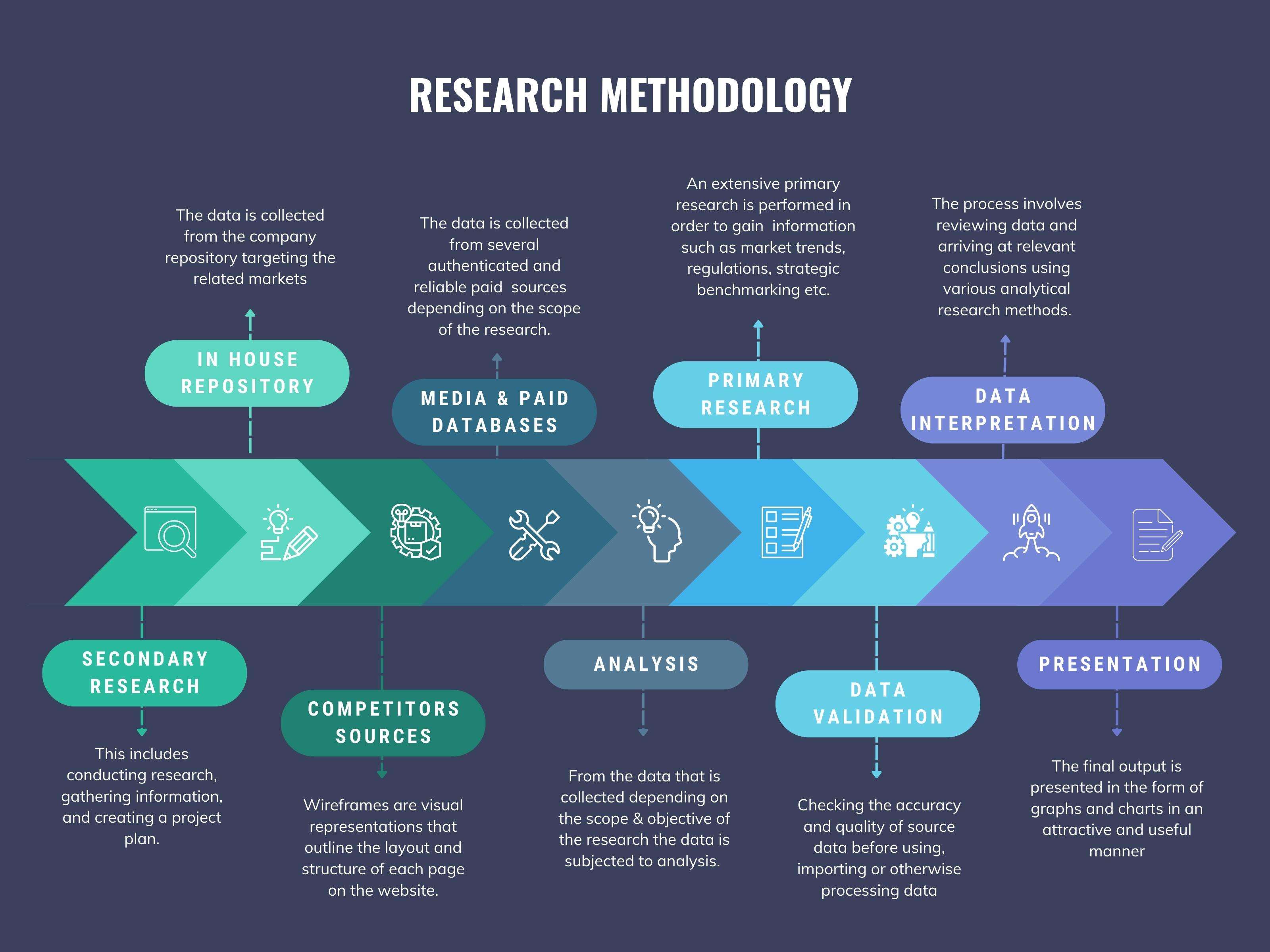
We at ‘Stratistics’ opt for an extensive research approach which involves data mining, data validation, and data analysis. The various research sources include in-house repository, secondary research, competitor’s sources, social media research, client internal data, and primary research.
Our team of analysts prefers the most reliable and authenticated data sources in order to perform the comprehensive literature search. With access to most of the authenticated data bases our team highly considers the best mix of information through various sources to obtain extensive and accurate analysis.
Each report takes an average time of a month and a team of 4 industry analysts. The time may vary depending on the scope and data availability of the desired market report. The various parameters used in the market assessment are standardized in order to enhance the data accuracy.
Data Mining
The data is collected from several authenticated, reliable, paid and unpaid sources and is filtered depending on the scope & objective of the research. Our reports repository acts as an added advantage in this procedure. Data gathering from the raw material suppliers, distributors and the manufacturers is performed on a regular basis, this helps in the comprehensive understanding of the products value chain. Apart from the above mentioned sources the data is also collected from the industry consultants to ensure the objective of the study is in the right direction.
Market trends such as technological advancements, regulatory affairs, market dynamics (Drivers, Restraints, Opportunities and Challenges) are obtained from scientific journals, market related national & international associations and organizations.
Data Analysis
From the data that is collected depending on the scope & objective of the research the data is subjected for the analysis. The critical steps that we follow for the data analysis include:
- Product Lifecycle Analysis
- Competitor analysis
- Risk analysis
- Porters Analysis
- PESTEL Analysis
- SWOT Analysis
The data engineering is performed by the core industry experts considering both the Marketing Mix Modeling and the Demand Forecasting. The marketing mix modeling makes use of multiple-regression techniques to predict the optimal mix of marketing variables. Regression factor is based on a number of variables and how they relate to an outcome such as sales or profits.
Data Validation
The data validation is performed by the exhaustive primary research from the expert interviews. This includes telephonic interviews, focus groups, face to face interviews, and questionnaires to validate our research from all aspects. The industry experts we approach come from the leading firms, involved in the supply chain ranging from the suppliers, distributors to the manufacturers and consumers so as to ensure an unbiased analysis.
We are in touch with more than 15,000 industry experts with the right mix of consultants, CEO's, presidents, vice presidents, managers, experts from both supply side and demand side, executives and so on.
The data validation involves the primary research from the industry experts belonging to:
- Leading Companies
- Suppliers & Distributors
- Manufacturers
- Consumers
- Industry/Strategic Consultants
Apart from the data validation the primary research also helps in performing the fill gap research, i.e. providing solutions for the unmet needs of the research which helps in enhancing the reports quality.
For more details about research methodology, kindly write to us at info@strategymrc.com
Frequently Asked Questions
In case of any queries regarding this report, you can contact the customer service by filing the “Inquiry Before Buy” form available on the right hand side. You may also contact us through email: info@strategymrc.com or phone: +1-301-202-5929
Yes, the samples are available for all the published reports. You can request them by filling the “Request Sample” option available in this page.
Yes, you can request a sample with your specific requirements. All the customized samples will be provided as per the requirement with the real data masked.
All our reports are available in Digital PDF format. In case if you require them in any other formats, such as PPT, Excel etc you can submit a request through “Inquiry Before Buy” form available on the right hand side. You may also contact us through email: info@strategymrc.com or phone: +1-301-202-5929
We offer a free 15% customization with every purchase. This requirement can be fulfilled for both pre and post sale. You may send your customization requirements through email at info@strategymrc.com or call us on +1-301-202-5929.
We have 3 different licensing options available in electronic format.
- Single User Licence: Allows one person, typically the buyer, to have access to the ordered product. The ordered product cannot be distributed to anyone else.
- 2-5 User Licence: Allows the ordered product to be shared among a maximum of 5 people within your organisation.
- Corporate License: Allows the product to be shared among all employees of your organisation regardless of their geographical location.
All our reports are typically be emailed to you as an attachment.
To order any available report you need to register on our website. The payment can be made either through CCAvenue or PayPal payments gateways which accept all international cards.
We extend our support to 6 months post sale. A post sale customization is also provided to cover your unmet needs in the report.
Request Customization
We offer complimentary customization of up to 15% with every purchase. To share your customization requirements, feel free to email us at info@strategymrc.com or call us on +1-301-202-5929. .
Please Note: Customization within the 15% threshold is entirely free of charge. If your request exceeds this limit, we will conduct a feasibility assessment. Following that, a detailed quote and timeline will be provided.
WHY CHOOSE US ?

Assured Quality
Best in class reports with high standard of research integrity

24X7 Research Support
Continuous support to ensure the best customer experience.

Free Customization
Adding more values to your product of interest.

Safe & Secure Access
Providing a secured environment for all online transactions.

Trusted by 600+ Brands
Serving the most reputed brands across the world.
