
Companion Diagnostics Market
Companion Diagnostics Market Forecasts to 2030 - Global Analysis By Product (Kits & Reagents and Assays), Indication (Leukaemia, Melanoma, Cancer, Neurological Disorders, Cardiovascular Diseases, Infectious Diseases and Other Indications), Technology, End User and By Geography

|
Years Covered |
2021-2030 |
|
Estimated Year Value (2023) |
US $7.24 BN |
|
Projected Year Value (2030) |
US $18.81 BN |
|
CAGR (2023 - 2030) |
14.6% |
|
Regions Covered |
North America, Europe, Asia Pacific, South America, and Middle East & Africa |
|
Countries Covered |
US, Canada, Mexico, Germany, UK, Italy, France, Spain, Japan, China, India, Australia, New Zealand, South Korea, Rest of Asia Pacific, South America, Argentina, Brazil, Chile, Middle East & Africa, Saudi Arabia, UAE, Qatar, and South Africa |
|
Largest Market |
North America |
|
Highest Growing Market |
Europe |
According to Stratistics MRC, the Global Companion Diagnostics Market is accounted for $7.24 billion in 2023 and is expected to reach $18.81 billion by 2030 growing at a CAGR of 14.6% during the forecast period. A companion diagnostic is a test used to ensure the usage of a comparable biological product or medication is both safe and effective. These tests are typically used to diagnose cancer, neurological, cardiovascular and infectious diseases. In this, an appropriate medicine is recommended based on the detected biomarker. Based on a particular patient's reaction, these tests offer tailored treatment. When deciding whether to use targeted therapeutics, companion diagnostic exams (CDXs) are crucial.
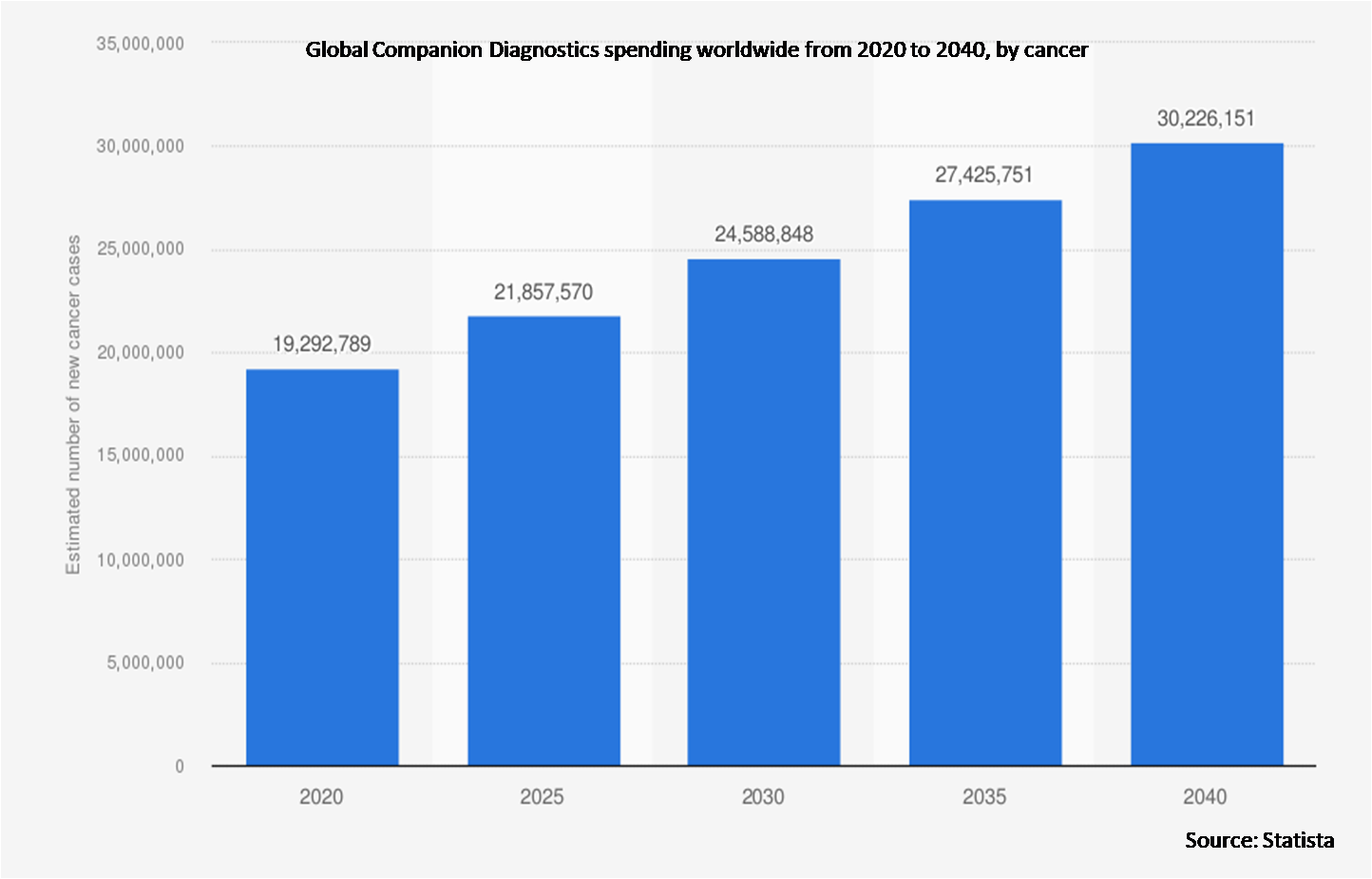
According to Lung Cancer Research Foundation report 2022, an estimated 236,740 people were diagnosed with lung cancer in 2022 in the United States, leading to more demand for lung cancer companion diagnostics.
Market Dynamics:
Driver:
Growing requirements for specialized treatments
Since genetic sequencing and genomics have advanced, it is generally accepted that various individuals might respond to medications differently. The technique of giving "the right drug, at the right time, at the right dose, for the right person" can be advanced by having a better understanding of a person's genetic traits or biomarkers. In order to give focused therapeutics to the appropriate candidate, pharmaceutical and biopharmaceutical firms are continually working to adopt patient-selection diagnostic frameworks in the early phases of drug development. This helps the market's expansion to grow further.
Restraint:
The instance for insufficient reimbursement
Despite growing acceptability, there are no established criteria for determining how much should be paid for a companion diagnostic test. Payment is determined on a case-by-case basis. For inpatient and outpatient care, reimbursement is different. Diagnostics are primarily covered for the inpatient care system by diagnosis-related groups. The payment is made in accordance with the detailed list of services that can be reimbursed for outpatient care. Therefore, the absence of compensation and onerous rules restrain market expansion.
Opportunity:
Increasing prevalence of cancer
The increased trends in new instances of pancreatic, female breast, and colorectal cancer as well as the stagnation of myeloma, uterine, and kidney cancer are attributed to the rising prevalence of obesity, diabetes, and physical inactivity. Companion diagnostics have proven to be a crucial tool for the treatment of individual patients in the clinic as well as for the process of developing new drugs. Information from companion diagnostics is crucial for the safe and efficient use of targeted medicines. This diagnosis has several ambitious objectives. The market is growing because of its effective diagnosis, monitoring, screening, and prognosis.
Threat:
High cost of immunotherapy treatment
The high expense of immunotherapy has restricted general patient access even though it has showed tremendous promise in the treatment of cancer. The length of the course of therapy might be increased from five months to over three years if doctors decide that it is beneficial to combine medicines. In this instance, the cost increases as the duration increases. Each CAR T-cell therapy is customized for the patient, requiring significant lab resources. As a result, the price per patient for the medicine alone rises to around USD 60,000. Cancer immune therapies can be far more expensive than other treatment choices like chemotherapy or radiation therapy. Consequently, it is anticipated that the high cost of immune-oncology treatments would restrain their expansion.
Covid-19 Impact
Early detection of COVID-19 became crucial in the market since it may aid in people with the condition receiving the right care. The polymerase chain reaction (PCR) test employing nasopharyngeal swabs or other upper respiratory tract specimens, including throat swabs, was the most widely used and trustworthy method for the diagnosis of COVID-19. Polymerase chain reaction (PCR)-based diagnostics, then, became a key area of focus for treating the illness. The development of new biomarkers for various diseases, an increase in research and development of targeted therapies, an increase in demand for customized medicine with increased recognition in developing markets, and a higher number of unmet medical needs are all contributing factors fuelled the market growth.
The cancer segment is expected to be the largest during the forecast period
The cancer segment is estimated to have a lucrative growth, due to the growing prevalence of lung cancer all around the world. When deciding whether to use targeted medicines to treat lung cancer, companion diagnostic tests (CDXs) are regarded as indispensable. Lung cancer patients who obtained companion diagnostics as part of their initial treatment had higher survival rates than patients who did not get screened. The increasing prevalence of non-small cell lung cancer (NSCLC), together with the acceleration of the development of oncology companion diagnostic tests for the condition, are expected to propel the segment's growth on a global scale.
The polymerase chain reaction (PCR) segment is expected to have the highest CAGR during the forecast period
The polymerase chain reaction (PCR) segment is anticipated to witness the fastest CAGR growth during the forecast period. The highly sensitive PCR method enables quick DNA amplification of a particular section. Using visual methods based on size and charge, PCR may detect and identify gene sequences by producing billions of copies of a certain DNA fragment or gene. It is a rapid diagnostic exam that takes 4 to 8 hours to complete. The category is growing as a consequence of its speedier results, efficient appropriate therapy, and anti-microbial resistance testing.
Region with largest share:
North America is projected to hold the largest market share during the forecast period. United States is emerging as a major contributor in the region. The United States has begun to play a significant role in this region. The most common users of companion diagnostics in the US are those with chronic diseases. The market is expanding as a result of rising research activities employing companion diagnostic kits in this region as well as the rising number of healthcare organizations working on genomic databases created to comprehend the human genome.
Region with highest CAGR:
Europe is projected to have the highest CAGR over the forecast period, owing to the growing preference for personalized medicine and increasing collaboration among companies. The region's highest market share is held by Germany and France. The market demand is being further fuelled by the region's growing medical tourism industry as well as the region's expanding senior population who are more susceptible to cancer and neurological illnesses.
Key players in the market
Some of the key players profiled in the Companion Diagnostics Market include Abbott, Agendia N.V, Agilent Technologies, ARUP Laboratories, Inc., Biogenex Laboratories, Inc., Dako, Inc., Roche Molecular Systems, Inc., Foundation Medicine, Inc., GE Healthcare, Genomic Health, Illumina, Inc., Genetron Holdings Limited, Labcorp Drug Development, Leica Biosystems, Life Technologies Corporation, MolecularMD Corporation, Myriad Genetic Laboratories, Inc., Ventana Medical Systems, Inc., Exact Sciences Corporation and Qiagen.
Key Developments:
In January 2023, Thermo Fisher Scientific partners with Astrazeneca to develop solid tissue and blood-based companion diagnostic test for tagrisso. The collaboration will leverage the Oncomine Dx Express Test* on the Genexus Dx System*, a fully-integrated next-generation sequencing (NGS) platform featuring an automated specimen-to-report workflow that economically delivers results in as little as 24 hours to bring test results to clinicians and patients faster.
In February 2022, Genetron Holdings Limited, a leading precision oncology platform company in China specializing in molecular profiling tests, early cancer screening products, and companion diagnostics development, entered a collaboration agreement with HUTCHMED (China) Limited to develop a companion diagnostic (CDx).
Products Covered:
• Kits & Reagents
• Assays
Indications Covered:
• Leukaemia
• Melanoma
• Cancer
• Neurological Disorders
• Cardiovascular Diseases
• Infectious Diseases
• Other Indications
Technologies Covered:
• Real-time PCR (RT-PCR)
• Polymerase Chain Reaction (PCR)
• Next-generation Sequencing
• In Situ Hybridization (ISH)
• Immunohistochemistry (IHC)
• Gene Sequencing
• Other Technologies
End Users Covered:
• Contract Research Organizations
• Pharmaceutical & Biopharmaceutical Companies
• Reference Laboratories
• Other End Users
Regions Covered:
• North America
o US
o Canada
o Mexico
• Europe
o Germany
o UK
o Italy
o France
o Spain
o Rest of Europe
• Asia Pacific
o Japan
o China
o India
o Australia
o New Zealand
o South Korea
o Rest of Asia Pacific
• South America
o Argentina
o Brazil
o Chile
o Rest of South America
• Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Rest of Middle East & Africa
What our report offers:
- Market share assessments for the regional and country-level segments
- Strategic recommendations for the new entrants
- Covers Market data for the years 2021, 2022, 2023, 2026, and 2030
- Market Trends (Drivers, Constraints, Opportunities, Threats, Challenges, Investment Opportunities, and recommendations)
- Strategic recommendations in key business segments based on the market estimations
- Competitive landscaping mapping the key common trends
- Company profiling with detailed strategies, financials, and recent developments
- Supply chain trends mapping the latest technological advancements
Free Customization Offerings:
All the customers of this report will be entitled to receive one of the following free customization options:
• Company Profiling
o Comprehensive profiling of additional market players (up to 3)
o SWOT Analysis of key players (up to 3)
• Regional Segmentation
o Market estimations, Forecasts and CAGR of any prominent country as per the client's interest (Note: Depends on feasibility check)
• Competitive Benchmarking
o Benchmarking of key players based on product portfolio, geographical presence, and strategic alliances
Table of Contents
1 Executive Summary
2 Preface
2.1 Abstract
2.2 Stake Holders
2.3 Research Scope
2.4 Research Methodology
2.4.1 Data Mining
2.4.2 Data Analysis
2.4.3 Data Validation
2.4.4 Research Approach
2.5 Research Sources
2.5.1 Primary Research Sources
2.5.2 Secondary Research Sources
2.5.3 Assumptions
3 Market Trend Analysis
3.1 Introduction
3.2 Drivers
3.3 Restraints
3.4 Opportunities
3.5 Threats
3.6 Product Analysis
3.7 Technology Analysis
3.8 End User Analysis
3.9 Emerging Markets
3.10 Impact of Covid-19
4 Porters Five Force Analysis
4.1 Bargaining power of suppliers
4.2 Bargaining power of buyers
4.3 Threat of substitutes
4.4 Threat of new entrants
4.5 Competitive rivalry
5 Global Companion Diagnostics Market, By Product
5.1 Introduction
5.2 Kits & Reagents
5.3 Assays
6 Global Companion Diagnostics Market, By Indication
6.1 Introduction
6.2 Leukaemia
6.3 Melanoma
6.4 Cancer
6.4.1 Lung Cancer
6.4.2 Breast Cancer
6.4.3 Blood Cancer
6.4.4 Colorectal Cancer
6.4.5 Other Cancer Types
6.5 Neurological Disorders
6.6 Cardiovascular Diseases
6.7 Infectious Diseases
6.8 Other Indications
7 Global Companion Diagnostics Market, By Technology
7.1 Introduction
7.2 Real-time PCR (RT-PCR)
7.3 Polymerase Chain Reaction (PCR)
7.4 Next-generation Sequencing
7.5 In Situ Hybridization (ISH)
7.6 Immunohistochemistry (IHC)
7.7 Gene Sequencing
7.8 Other Technologies
8 Global Companion Diagnostics Market, By End User
8.1 Introduction
8.2 Contract Research Organizations
8.3 Pharmaceutical & Biopharmaceutical Companies
8.4 Reference Laboratories
8.5 Other End Users
9 Global Companion Diagnostics Market, By Geography
9.1 Introduction
9.2 North America
9.2.1 US
9.2.2 Canada
9.2.3 Mexico
9.3 Europe
9.3.1 Germany
9.3.2 UK
9.3.3 Italy
9.3.4 France
9.3.5 Spain
9.3.6 Rest of Europe
9.4 Asia Pacific
9.4.1 Japan
9.4.2 China
9.4.3 India
9.4.4 Australia
9.4.5 New Zealand
9.4.6 South Korea
9.4.7 Rest of Asia Pacific
9.5 South America
9.5.1 Argentina
9.5.2 Brazil
9.5.3 Chile
9.5.4 Rest of South America
9.6 Middle East & Africa
9.6.1 Saudi Arabia
9.6.2 UAE
9.6.3 Qatar
9.6.4 South Africa
9.6.5 Rest of Middle East & Africa
10 Key Developments
10.1 Agreements, Partnerships, Collaborations and Joint Ventures
10.2 Acquisitions & Mergers
10.3 New Product Launch
10.4 Expansions
10.5 Other Key Strategies
11 Company Profiling
11.1 Abbott
11.2 Agendia N.V
11.3 Agilent Technologies
11.4 ARUP Laboratories, Inc.
11.5 Biogenex Laboratories, Inc.
11.6 Dako, Inc.
11.7 Roche Molecular Systems, Inc.
11.8 Thermo Fisher Scientific
11.9 GE Healthcare
11.10 Genomic Health
11.11 Illumina, Inc.
11.12 Genetron Holdings Limited
11.13 Labcorp Drug Development
11.14 Leica Biosystems
11.15 Life Technologies Corporation
11.16 MolecularMD Corporation
11.17 Myriad Genetic Laboratories, Inc.
11.18 Ventana Medical Systems, Inc.
11.19 Exact Sciences Corporation
11.20 Qiagen
List of Tables
1 Global Companion Diagnostics Market Outlook, By Region (2021-2030) ($MN)
2 Global Companion Diagnostics Market Outlook, By Product (2021-2030) ($MN)
3 Global Companion Diagnostics Market Outlook, By Kits & Reagents (2021-2030) ($MN)
4 Global Companion Diagnostics Market Outlook, By Assays (2021-2030) ($MN)
5 Global Companion Diagnostics Market Outlook, By Indication (2021-2030) ($MN)
6 Global Companion Diagnostics Market Outlook, By Leukaemia (2021-2030) ($MN)
7 Global Companion Diagnostics Market Outlook, By Melanoma (2021-2030) ($MN)
8 Global Companion Diagnostics Market Outlook, By Cancer (2021-2030) ($MN)
9 Global Companion Diagnostics Market Outlook, By Lung Cancer (2021-2030) ($MN)
10 Global Companion Diagnostics Market Outlook, By Breast Cancer (2021-2030) ($MN)
11 Global Companion Diagnostics Market Outlook, By Blood Cancer (2021-2030) ($MN)
12 Global Companion Diagnostics Market Outlook, By Colorectal Cancer (2021-2030) ($MN)
13 Global Companion Diagnostics Market Outlook, By Other Cancer Types (2021-2030) ($MN)
14 Global Companion Diagnostics Market Outlook, By Neurological Disorders (2021-2030) ($MN)
15 Global Companion Diagnostics Market Outlook, By Cardiovascular Diseases (2021-2030) ($MN)
16 Global Companion Diagnostics Market Outlook, By Infectious Diseases (2021-2030) ($MN)
17 Global Companion Diagnostics Market Outlook, By Other Indications (2021-2030) ($MN)
18 Global Companion Diagnostics Market Outlook, By Technology (2021-2030) ($MN)
19 Global Companion Diagnostics Market Outlook, By Real-time PCR (RT-PCR) (2021-2030) ($MN)
20 Global Companion Diagnostics Market Outlook, By Polymerase Chain Reaction (PCR) (2021-2030) ($MN)
21 Global Companion Diagnostics Market Outlook, By Next-generation Sequencing (2021-2030) ($MN)
22 Global Companion Diagnostics Market Outlook, By In Situ Hybridization (ISH) (2021-2030) ($MN)
23 Global Companion Diagnostics Market Outlook, By Immunohistochemistry (IHC) (2021-2030) ($MN)
24 Global Companion Diagnostics Market Outlook, By Gene Sequencing (2021-2030) ($MN)
25 Global Companion Diagnostics Market Outlook, By Other Technologies (2021-2030) ($MN)
26 Global Companion Diagnostics Market Outlook, By End User (2021-2030) ($MN)
27 Global Companion Diagnostics Market Outlook, By Contract Research Organizations (2021-2030) ($MN)
28 Global Companion Diagnostics Market Outlook, By Pharmaceutical & Biopharmaceutical Companies (2021-2030) ($MN)
29 Global Companion Diagnostics Market Outlook, By Reference Laboratories (2021-2030) ($MN)
30 Global Companion Diagnostics Market Outlook, By Other End Users (2021-2030) ($MN)
Note: Tables for North America, Europe, APAC, South America, and Middle East & Africa Regions are also represented in the same manner as above.
List of Figures
RESEARCH METHODOLOGY
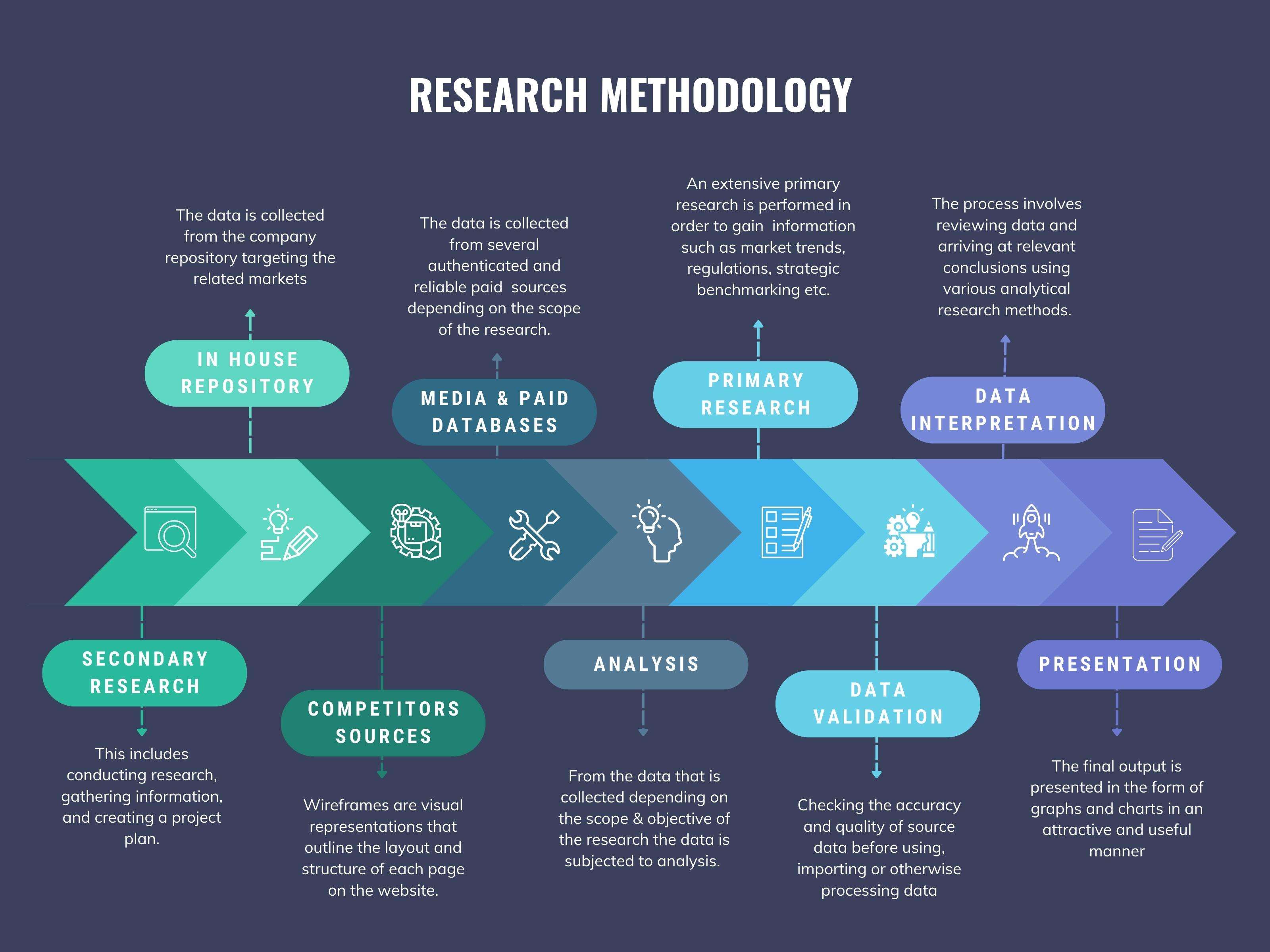
We at ‘Stratistics’ opt for an extensive research approach which involves data mining, data validation, and data analysis. The various research sources include in-house repository, secondary research, competitor’s sources, social media research, client internal data, and primary research.
Our team of analysts prefers the most reliable and authenticated data sources in order to perform the comprehensive literature search. With access to most of the authenticated data bases our team highly considers the best mix of information through various sources to obtain extensive and accurate analysis.
Each report takes an average time of a month and a team of 4 industry analysts. The time may vary depending on the scope and data availability of the desired market report. The various parameters used in the market assessment are standardized in order to enhance the data accuracy.
Data Mining
The data is collected from several authenticated, reliable, paid and unpaid sources and is filtered depending on the scope & objective of the research. Our reports repository acts as an added advantage in this procedure. Data gathering from the raw material suppliers, distributors and the manufacturers is performed on a regular basis, this helps in the comprehensive understanding of the products value chain. Apart from the above mentioned sources the data is also collected from the industry consultants to ensure the objective of the study is in the right direction.
Market trends such as technological advancements, regulatory affairs, market dynamics (Drivers, Restraints, Opportunities and Challenges) are obtained from scientific journals, market related national & international associations and organizations.
Data Analysis
From the data that is collected depending on the scope & objective of the research the data is subjected for the analysis. The critical steps that we follow for the data analysis include:
- Product Lifecycle Analysis
- Competitor analysis
- Risk analysis
- Porters Analysis
- PESTEL Analysis
- SWOT Analysis
The data engineering is performed by the core industry experts considering both the Marketing Mix Modeling and the Demand Forecasting. The marketing mix modeling makes use of multiple-regression techniques to predict the optimal mix of marketing variables. Regression factor is based on a number of variables and how they relate to an outcome such as sales or profits.
Data Validation
The data validation is performed by the exhaustive primary research from the expert interviews. This includes telephonic interviews, focus groups, face to face interviews, and questionnaires to validate our research from all aspects. The industry experts we approach come from the leading firms, involved in the supply chain ranging from the suppliers, distributors to the manufacturers and consumers so as to ensure an unbiased analysis.
We are in touch with more than 15,000 industry experts with the right mix of consultants, CEO's, presidents, vice presidents, managers, experts from both supply side and demand side, executives and so on.
The data validation involves the primary research from the industry experts belonging to:
- Leading Companies
- Suppliers & Distributors
- Manufacturers
- Consumers
- Industry/Strategic Consultants
Apart from the data validation the primary research also helps in performing the fill gap research, i.e. providing solutions for the unmet needs of the research which helps in enhancing the reports quality.
For more details about research methodology, kindly write to us at info@strategymrc.com
Frequently Asked Questions
In case of any queries regarding this report, you can contact the customer service by filing the “Inquiry Before Buy” form available on the right hand side. You may also contact us through email: info@strategymrc.com or phone: +1-301-202-5929
Yes, the samples are available for all the published reports. You can request them by filling the “Request Sample” option available in this page.
Yes, you can request a sample with your specific requirements. All the customized samples will be provided as per the requirement with the real data masked.
All our reports are available in Digital PDF format. In case if you require them in any other formats, such as PPT, Excel etc you can submit a request through “Inquiry Before Buy” form available on the right hand side. You may also contact us through email: info@strategymrc.com or phone: +1-301-202-5929
We offer a free 15% customization with every purchase. This requirement can be fulfilled for both pre and post sale. You may send your customization requirements through email at info@strategymrc.com or call us on +1-301-202-5929.
We have 3 different licensing options available in electronic format.
- Single User Licence: Allows one person, typically the buyer, to have access to the ordered product. The ordered product cannot be distributed to anyone else.
- 2-5 User Licence: Allows the ordered product to be shared among a maximum of 5 people within your organisation.
- Corporate License: Allows the product to be shared among all employees of your organisation regardless of their geographical location.
All our reports are typically be emailed to you as an attachment.
To order any available report you need to register on our website. The payment can be made either through CCAvenue or PayPal payments gateways which accept all international cards.
We extend our support to 6 months post sale. A post sale customization is also provided to cover your unmet needs in the report.
Request Customization
We provide a free 15% customization on every purchase. This requirement can be fulfilled for both pre and post sale. You may send your customization requirements through email at info@strategymrc.com or call us on +1-301-202-5929.
Note: This customization is absolutely free until it falls under the 15% bracket. If your requirement exceeds this a feasibility check will be performed. Post that, a quote will be provided along with the timelines.
WHY CHOOSE US ?

Assured Quality
Best in class reports with high standard of research integrity

24X7 Research Support
Continuous support to ensure the best customer experience.

Free Customization
Adding more values to your product of interest.

Safe & Secure Access
Providing a secured environment for all online transactions.

Trusted by 600+ Brands
Serving the most reputed brands across the world.
